Calcified coronary artery lesions are one of the most challenging lesions to treat and are associated with worse clinical outcomes.1 Initially, rotablation or orbital atherectomy were the only treatment options available, with both being associated with a steep learning curve and difficult-to-treat complications. With time, balloon-based devices have evolved and intravascular lithotripsy (IVL) has come into the fore.2 However, any single technology may not be enough, and, in some cases, the complementary use of balloon-based calcium ablators, such as IVL, and rotational or orbital atherectomy may be needed for optimal plaque preparation and stent deployment.3
Here, we describe three cases in which the complementary use of two technologies helped successfully manage calcified coronary artery lesions.
Case Presentation
Case 1
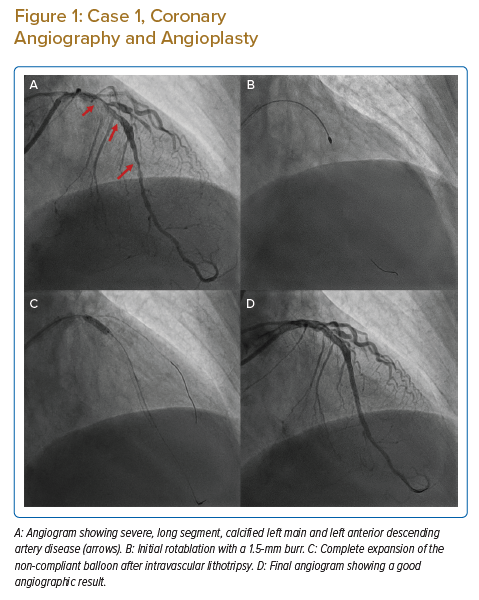
A 77-year-old man, with known diabetes, hypertension and chronic kidney disease, presented with accelerating angina over the previous 9 days. The patient had severe left ventricular dysfunction, with an ejection fraction of 35% and severe global hypokinesia. Coronary angiography revealed severely calcified left main (LM) and diffuse left anterior descending artery (LAD) disease (Figure 1A) and mid-right coronary artery (RCA) chronic total occlusion. He had a SYNTAX score of 40 and a EuroSCORE II of 18.45%.
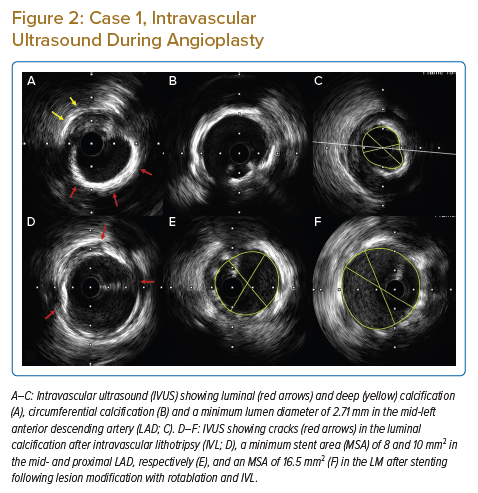
The patient underwent intra-aortic balloon pump (IABP)-supported percutaneous intervention (PCI) with a plan for rotational atherectomy to mid-LAD lesion followed by IVL, if required. Initial rotablation was successfully performed in the proximal and mid-LAD with a 1.5 mm burr (see Figure 1B). After rotablation, intravascular ultrasonography (IVUS) revealed an arc of thick circumferential calcium in the LM and proximal LAD, along with deep medial calcification in the LM and LAD (Figures 2A–2C). After IVUS, a 3 mm NC balloon could not be expanded, even at 20 atm (Figure 1B). Considering the large reference minimum lumen diameter (MLD) and thick circumferential calcium, the next best option was to use an IVL or a very-high-pressure OPN balloon. We decided to use a 3.5 mm IVL balloon because the calcium appeared very thick and we considered IVL safer than using a very-high-pressure OPN balloon.
IVL was successfully performed with 3.5 mm × 12 mm balloon with 40 pulses delivered in LM and 40 pulses in the proximal LAD. The patient tolerated prolonged balloon inflation in the LM despite poor left ventricular function because he also had IABP support. We deployed a cross-over 3.5 mm × 38 mm zotarolimus-eluting stent from the LM to proximal LAD, followed by proximal optimisation (POT) of LM stent with 4.5 mm and 5 mm non-compliant (NC) balloons. We deployed a 2.5 mm × 28 mm everolimus-eluting stent from the mid- to distal LAD with final post-dilation with a 3.0 mm NC balloon.
Post-stent IVUS revealed multiple cracks in the calcium arc (Figure 2D) with an LM minimum stent area (MSA) of 16.5 mm2 (Figure 2F), and proximal and mid-LAD MSAs of 10 mm2 and 8 mm2, respectively (Figure 2E), with good stent apposition (Figure 1D). The patient’s IABP was removed the next day and was discharged after 2 days. The patient is currently asymptomatic at 1-year follow-up.
Case 2
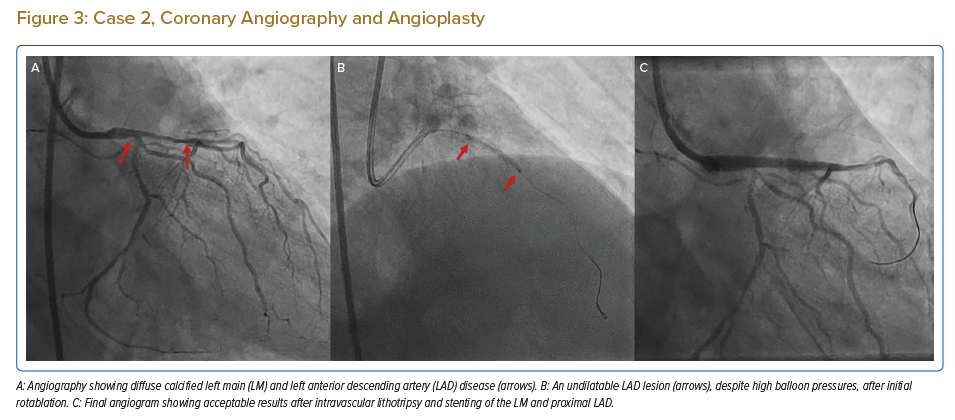
A 75-year-old man with diabetes, hypertension, severe Parkinson’s disease and coronary artery disease presented to the emergency department with severe angina. Coronary angiography revealed severely calcified LM and LAD disease, with 70% stenosis of the distal LM and a diffusely diseased, very tortuous proximal LAD with diffuse 80% stenosis (Figure 3A). The distal left circumflex artery (LCx) had 70% stenosis, and the RCA had mild disease. His SYNTAX score was 33 and his EuroSCORE II was 13.07.
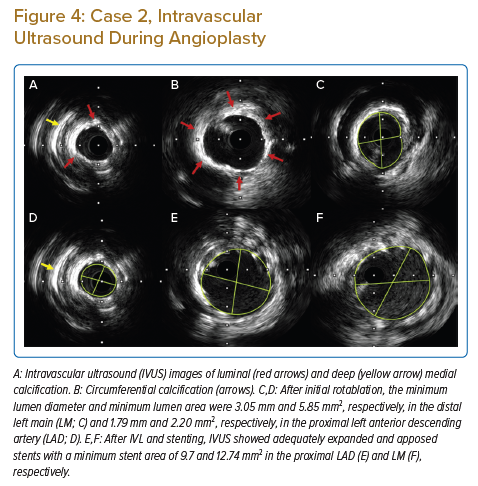
The patient was taken up for PCI with rotablation and IVL, if required. An initial rotablation was performed with a small, 1.25 mm burr in the distal LM and proximal LAD due to severe tortuosity. IVUS after rotablation revealed circumferential calcium on the luminal surface along with deep medial calcification (Figures 4A and 4B). Although luminal calcium was modified by rotablation, the MLD and minimum lumen area (MLA) remained low in the proximal LAD and distal LM (Figures 4C and 4D) and the lesion could not be dilated with 3 mm NC balloon, even at high pressures (Figure 3B), due to heavy medial calcification.
Hence, we proceeded with IVL. IVL was performed with a 3 mm balloon, with 40 pulses given in the proximal LAD and another 40 pulses in the distal LM. After IVL, the lesions were easily modified with a 3 mm × 15 mm NC balloon followed by stenting with a zotarolimus-eluting stent (3.5 mm × 22 mm) from the LM to LAD and an everolimus-eluting stent (2.25 mm × 24 mm) in the proximal to mid-LAD. The LAD stent was post-dilated with a 3.25-mm NC balloon and LM POT was done with 4 mm and 4.5 mm NC balloons. Post-stenting IVUS revealed an MSA of 12.74 mm2 with 9.7 mm2 and 9.7 mm2 in the LM and proximal LAD, respectively, with good apposition and expansion (Figures 3C,4E and 4F). The patient was discharged on day 2 and continues to do well at the 10-month follow-up.
Case 3
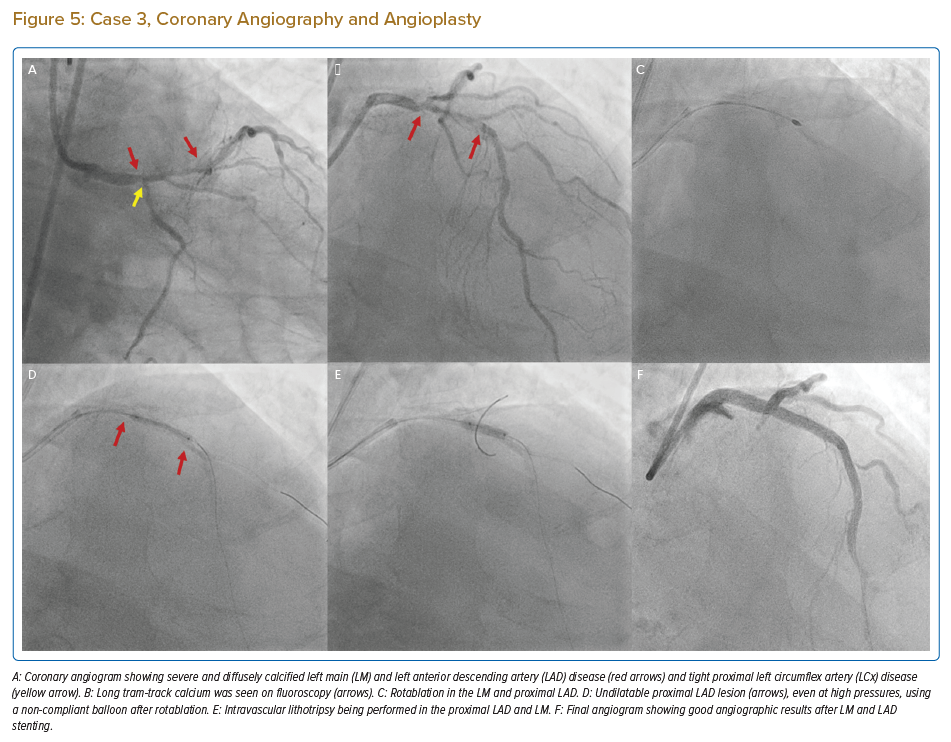
An 82-year-old man with diabetes, hypertension and chronic kidney disease who had been medically managed for chronic stable angina for the past 6 years presented with rest angina for 4 days. Coronary angiography revealed heavily calcified critical LM disease, diffusely diseased and calcified proximal LAD disease, tight proximal LCx disease (Figure 5A–C), dominant RCA with mid-diffuse disease and chronic total occlusion of the right posterior descending artery, with collaterals from the LAD, and an ejection fraction of 40% with inferior wall akinesia. His SYNTAX score was 36 and his EuroSCORE II was 14.85.
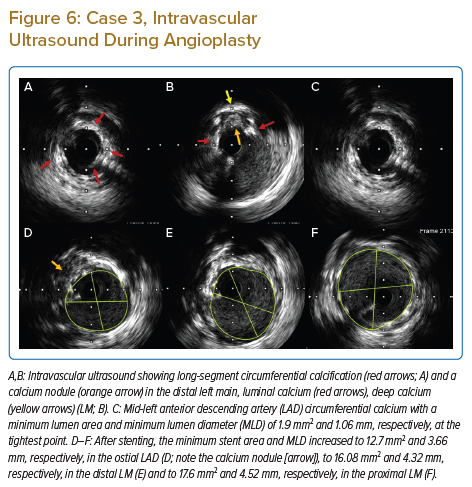
The patient was taken up for high-risk ‘protected’ PCI; an IABP was inserted and initial rotablation was performed using a 1.5 mm burr in the LM, proximal and mid-LAD. After rotablation, IVUS revealed circumferential diffuse calcification in the proximal to mid-LAD and a calcified nodule in the distal LM (Figures 6A–6C). A 2.5 mm NC balloon failed to dilate fully even at 24 atm, in the mid- and proximal LAD lesions (Figure 5D). Due to very heavy calcification and a large MLD after initial rotablation and the high risk of slow/no reflow with a larger-sized rotablator device, we decided to perform IVL.
Successful IVL was performed with 3 mm and 3.5 mm balloons in the proximal to mid-distal LAD and distal LM, respectively (Figure 5E). While performing IVL with a 3 mm balloon in the ostial LCx lesion, the balloon ruptured after 20 pulses, causing flow-limiting dissection in the LM. The reason for IVL balloon rupture was not clear, but it was probably due to nodular calcium at this location.
Emergency LM bifurcation stenting was done with a minicrush technique and the LM ostium to LAD was stented with a zotarolimus-eluting stent (3.5 mm × 26 mm), with a 3 mm × 26 mm zotarolimus-eluting stent deployed from the ostium to mid-LCx. LM POT was performed using a 5 mm NC balloon followed by final kissing balloon inflation with 3.5 mm and 3 mm NC balloons in the LAD and LCx. The mid- to distal LAD was successfully stented with two overlapping stents.
Post stenting IVUS images of LM and proximal LAD are shown in Figures 6D–6F and final angiographic result is in Figure 5F. The IABP was removed 24 hours after the procedure and the patient was discharged after 72 hours. The patient is currently asymptomatic during daily activities and moderate exertion.
Discussion
Calcified coronary stenoses are one of the most difficult lesions to treat and are associated with worse clinical outcomes.1 Currently, there are several devices available for dealing with calcified lesions.3 Balloon-based devices include scoring balloons, cutting balloons, super-high-pressure NC balloons and IVL. Ablation-based devices include rotablation and orbital atherectomy.
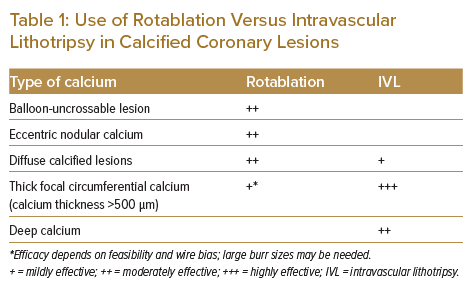
Although rotablation has been available for the treatment of coronary lesions for the past three decades, it is not commonly used because of the steep learning curve, its ability to modify only luminal calcium and difficulty in managing complications related to its use.4 These considerations have prompted research for alternative modalities, such as IVL. The main advantages of IVL are that it is easy to use, it can modify deep calcium and its use is associated with less chance of slow/no reflow and fewer coronary perforations.2 Rotablation and IVL can be used together in cases where IVL cannot cross a very tight stenosis, in cases of eccentric calcified nodules or long diffuse disease, where multiple balloons may be required.5–7
In all three cases described above, we performed upfront rotablation to ablate luminal calcium because the lesions were long, diffuse or nodular. In all three cases, IVUS was performed only after initial rotablation because IVUS could not cross the lesion before initial plaque modification. In this scenario, upfront use of the rotablator with a small burr is the best choice and IVL can be performed later depending on IVUS findings and/or results of initial balloon dilatation, as in all three cases described in this report. Rotablation with a small burr is safe but sometimes insufficient in terms of significantly modifying plaque, especially in large vessels with significant deep calcium and if the calcium thickness is >500 µm.8
Using a larger-sized burr may be effective in decreasing the calcium volume and helping to make calcium cracks with further balloon dilatation, but can be associated with risks such as slow/no reflow or coronary perforation if there is unfavourable wire bias or significant vessel tortuosity. This is where its combination of rotablation with IVL is very effective, because IVL is very efficient in treating large vessels with deep and circumferential calcification (Table 1). Furthermore, the upfront use of IVL in diffuse calcified lesions can be challenging because the IVL balloon has a large crossing profile. In addition, in lesions with long diffuse calcified disease, more than one IVL balloon may be required, which may be cost prohibitive.
It has also been observed that the upfront use of IVL in eccentric calcified nodules may lead to IVL balloon rupture, as occurred in our Case 3 and previously reported.9 A large rotablator burr or orbital atherectomy remain the best modalities to modify eccentric nodular calcium if there is favourable wire bias.
Combining upfront rotablation and IVL has several advantages. Initial rotablation can treat the diffuse intimal calcium and/or calcified nodule and IVL can be selectively used later in lesions with circumferential and/or deep calcium, which are not modified with balloon dilatation after initial rotablation. Initial rotablation may also prevent IVL balloon rupture, as has been reported previously.9 In addition, prior rotablation favourably modifies the vessel for the easy delivery of the lithotripsy balloon and other devices.5
Ideally, diffuse calcified lesions should be treated with imaging guidance. Calculation of the calcium score can provide guidance as to the need for advanced plaque preparation techniques, such as atherectomy or IVL.10,11 Imaging also helps define calcium morphology, such as eccentricity, nodularity, thickness, length and location, especially at bifurcations, to enable further planning of plaque preparation strategies.12
Optical coherence tomography has the advantage of providing calcium thickness, but its use may be limited in patients with compromised renal function or LM disease due to poor visualisation of the ostium of the LM.13
For these reasons, IVUS was chosen as the imaging modality in all three cases described here.
Conclusion
IVL and rotablation are complementary technologies for the treatment of diffuse calcified lesions. Upfront rotablation with a small-sized burr is safe and favourably modifies the superficial calcium, also helping in the efficient delivery of the IVL balloon, if required later.
Upfront use of IVL in the case of diffuse calcified lesions may be unsuccessful or associated with complications.
Clinical Perspective
- Intravascular lithotripsy (IVL) works best in concentric calcified lesions and is very effective in the case of thick and focal circumferential calcified lesions.
- Rotablation is most effective for nodular calcified lesions and lesions that a balloon cannot cross.
- The combination of rotablation and IVL is very effective in diffuse calcified lesions, with the initial rotablation modifying superficial calcium and helping in the efficient delivery of the IVL balloon, which can further modify deep and thick calcium, if required.
- Upfront use of IVL in the case of diffuse calcified lesions should probably be avoided without imaging so that it is used more effectively and prevents complications such as balloon rupture.