In India, the prevalence of rheumatic heart disease in the general population is 1.5–2.0 per 1,000.1,2 However, based on echocardiography, the prevalence of rheumatic heart disease is likely to be greater.3 The prevalence of Wolff–Parkinson–White (WPW) syndrome or the WPW pattern in the general population ranges from 0.1 to 0.3%, with a higher prevalence among men than women.4 The coexistence of rheumatic mitral stenosis and WPW syndrome is so rare that a search of the literature using Google Scholar and PubMed yielded 17 cases between 1960 and 2021. Among these, balloon mitral valvotomy and radiofrequency ablation were performed simultaneously in only two cases.5,6
Case Report
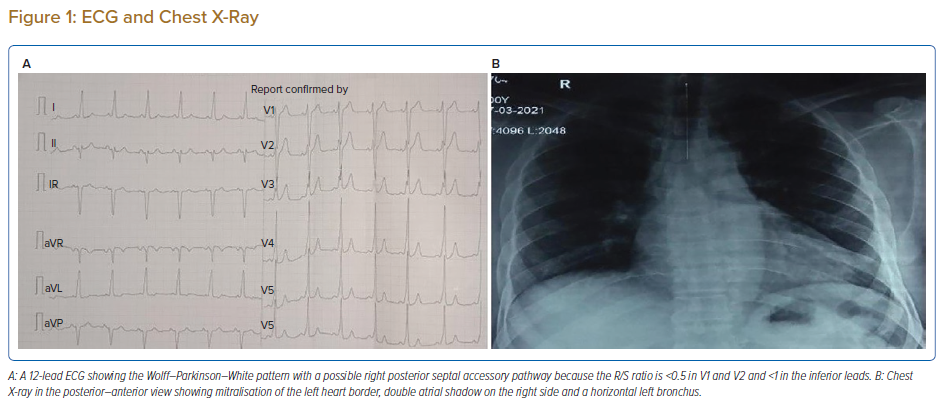
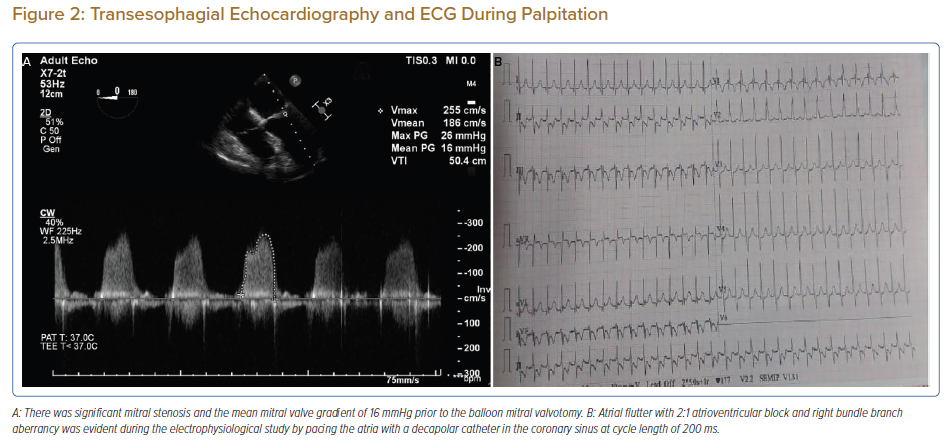
A 57-year-old man presented with a three-decade history of recurrent episodes of palpitation with alarming jugular venous pulsation, but without syncope. Each episode spontaneously reverted to the normal sinus rhythm 6–8 hours after the onset. Cardiac auscultation raised the suspicion of underlying rheumatic mitral stenosis. A 12-lead ECG was suggestive of a WPW pattern with a right posterior septal accessory pathway (Figure 1A). A chest X-ray in the posterior–anterior view was consistent with cardiac auscultation (Figure 1B). A transoesophageal echocardiogram confirmed rheumatic mitral stenosis (Figure 2A and Supplementary Material Video 1). The pliable mitral valve area was 0.8 cm2 and the mean gradient was 17 mmHg at a heart rate of 87 BPM. The coronary angiogram was normal.
An electrophysiologist, cardiothoracic surgeon, cardiac anaesthetist and cardiologist suggested mitral valvotomy followed by ablation of the accessory pathway in a single procedure if possible to avoid repeated septal puncture. Informed consent was obtained for the procedure.
The day before the procedure, the patient developed an episode of palpitation during the clinical round. A 12-lead ECG revealed atrial flutter with right bundle branch aberrancy on metoprolol succinate (Figure 2B). The patient’s blood pressure was 124/80 mmHg. Oral verapamil was initiated and the atrial flutter reverted to normal sinus rhythm.
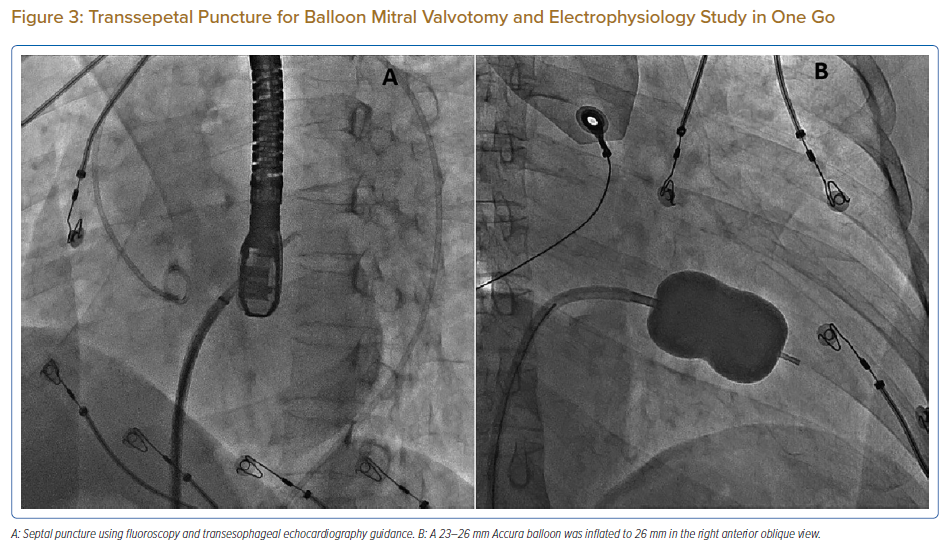
On the day of the procedure, the first balloon mitral valvotomy was performed from a right femoral approach using a 23–26 mm Accura balloon (Vascular Concepts) after transeptal access using an 8 Fr SL-1 sheath and a BRK-0 needle (St Jude Medical). A transeptal puncture was performed after proper needle tip position was confirmed by fluoroscopy (right anterior oblique, left anterior oblique and 90° lateral views) and transoesophageal echocardiography (bicaval and short axis views; Figure 3A). The mean left atrial pressure prior to the valvotomy was 31 mmHg. The balloon was inflated to 26 mm in the right anterior oblique 20° position under fluoroscopy (Figure 3B) because the patient was 160 cm tall. The mitral valve area increased to 2.2 cm2 without any additional mitral regurgitation, and the mean left atrial pressure decreased to 12 mmHg without any mitral valve gradient. Immediate transthoracic echocardiography showed that the mitral valve gradient had decreased to 7/2 mmHg with negligible mitral regurgitation.
The left atrial wire was reintroduced into the left atrium before the stretched balloon was removed from the left atrium for the electrophysiological study and for possible radiofrequency ablation (Figure 3C). The electrophysiologist proceeded with the ablation plan.
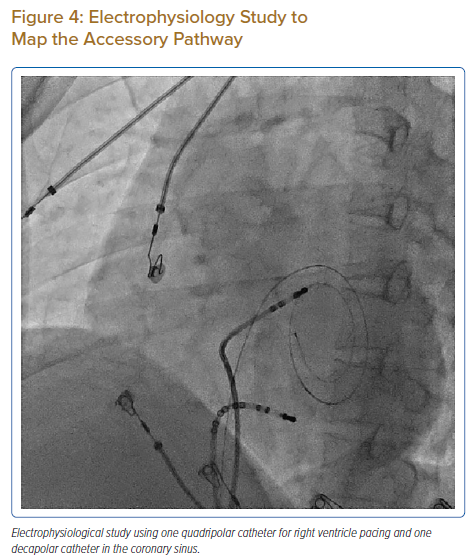
Because the patient had baseline pre-excitation through the right posterior septal path and atrial flutter with orthodromic conduction with right bundle branch aberration, one decapolar catheter in the coronary sinus and a quadripolar catheter in the right ventricle were used to study the effective refractory period (ERP) of the accessory pathway rather than using the routine four electrophysiology catheters (three quadripolar [high right atrial, His bundle, right ventricle apex] and one decapolar catheter in the coronary sinus; Figure 4C). The supra-His conduction time was 65 ms and the infra-His conduction time was 9 ms. The baseline ECG was suggestive of a right posterior septal pathway. Right ventricular pacing showed eccentric conduction up to 450 ms, which suggested a retrograde pathway ERP of 450 ms. On pacing the integrated pathway, the ERP was found to be 450 ms. Due to the weak nature of the accessory pathway, ablation was not performed. On rapid atrial pacing, atrial flutter with right bundle branch aberrancy was induced, similar to the clinical tachycardia observed earlier. Ablation for the atrial flutter was not performed, and the patient was maintained on metoprolol succinate and an oral anticoagulant in the anticipation of spontaneous remission of atrial flutter after both remodelling of the atrium and haemodynamic improvement after balloon valvotomy.
This patient has remained asymptomatic over a follow-up period of 15 months.
Discussion
Balloon mitral valvotomy is preferred to surgery in the case of pliable rheumatic stenosis. Of the treatments available for valvular AF, less is known about the efficacy of radiofrequency ablation because of the lack of a significant number of randomised control trials.7 Arrhythmia-related death in asymptomatic pre-excitation is as low as 0.05 to 0.9 per 1,000.8 Therefore, the treatment of rheumatic mitral stenosis with bystander involvement of an accessory pathway and an ERP that is greater than that of the atrioventricular node is not a challenge. Ablation of the accessory pathway is not indicated if the bystander pathway has a high ERP.9 In the present case, the patient had recurrent episodes of palpitation, but the right posterior septal accessory pathway did not contribute to these, which is quite an unusual scenario and unlike the case reported by Jagadheesan et al.10
Patients with rheumatic mitral stenosis who routinely seek help for fibrillation or flutter are in the 30- to 50-year age group, but the coexistence of a bystander right posterior septal accessory pathway, as in the present case, is unusual.11
The incidence of AF in a patient with an accessory pathway is 10–38%, but the association with common atrial flutter is not known.12 Our patient had orthodromic conduction of atrial flutter through the atrioventricular node because the coexisting right posterior septal pathway had an ERP of ≥450 ms. Neither the atrial flutter nor right posterior septal pathway were ablated, with the expectation that, during follow-up, both would become non-functional over time because of left atrial remodelling and favourable haemodynamic changes.13 It is well established that mitral stenosis causes AF, and the incidence of AF is higher in older age groups. It is also known that the results of balloon mitral valvotomy are worse in older patients because of persistent AF.14 Therefore, it has been suggested that balloon mitral valvotomy is performed at an early age for favourable atrial remodelling to reduce the occurrence of AF or atrial flutter.15
Conclusion
Palpitations caused by atrial flutter with right bundle branch aberrancy in a patient with rheumatic mitral stenosis and a right posterior septal accessory pathway with an ERP higher than that of the atrioventricular node are rare. Whether left atrial remodelling after percutaneous balloon mitral valvotomy further reduces atrial flutter requires additional investigation in larger studies with a longer follow-up period.
Clinical Perspective
- Severe rheumatic mitral stenosis associated with a right posterior septal accessory pathway is rare.
- It is very unusual that recurrent palpitations are caused by atrial flutter with right bundle branch aberrancy; rather, palpitations are likely caused by right posterior septal accessory pathway-mediated Wolff–Parkinson–White syndrome because the right posterior septal accessory pathway has a lower effective refractory period than the atrioventricular node.
- Treating both conditions with a single intervention (i.e. by balloon mitral valvotomy and radiofrequency ablation) is rare.
- The almost complete resolution of atrial flutter 15 months after balloon mitral valvotomy in this patient is an interesting finding.