Drug-coated balloons (DCBs) represent a method of revascularisation of coronary artery disease without requiring a permanent stent implant, which could induce an inflammatory response and delayed healing. Following on from studies demonstrating the inferiority of bioresorbable scaffolds as compared with drug-eluting stents (DESs) in terms of safety and major adverse cardiac events (MACE), DCBs represent the only feasible method currently available to achieve revascularisation without the requirement for permanent implantation.1 The DCB is a semi-compliant balloon coated with anti-proliferative drugs, which are released quickly when in contact with the vessel wall.2 In the right clinical scenario, the DCB has good long-term results with a low rate of restenosis without the inherent risk of stent thrombosis or in-stent restenosis.3
A recent update on the usage of DCB by the International DCB Consensus Group evaluated the contemporary literature on DCB usage and described the various scenarios in which DCBs can potentially be used.4
Despite the promising results of DCBs, their adoption in clinical practice has been slow and varies widely between different countries in the Asia-Pacific region. This formed the basis of the questionnaire upon which this paper is constructed. The primary aim of this study was to understand the pattern of DCB usage among interventionists in the Asia-Pacific region when faced with specific scenarios during percutaneous coronary intervention (PCI) and to review the awareness of the DCB expert consensus paper.
Methods
We designed a questionnaire to review the awareness of the DCB expert consensus paper and understand the prevalence of DCB usage in various scenarios faced commonly during PCI. This questionnaire was sent as an online link to actively practising interventional cardiologists in the Asia-Pacific region in collaboration with the Asian Pacific Society of Cardiology. Mailers include links within social media channels, society website and direct email. Responses with missing data were excluded (one response).
SPSS 16.0 software was used to perform the statistical analysis, and the level of statistical significance was set at p≤0.05. χ2 tests and paired T-tests were used to study the correlation between variables.
Results
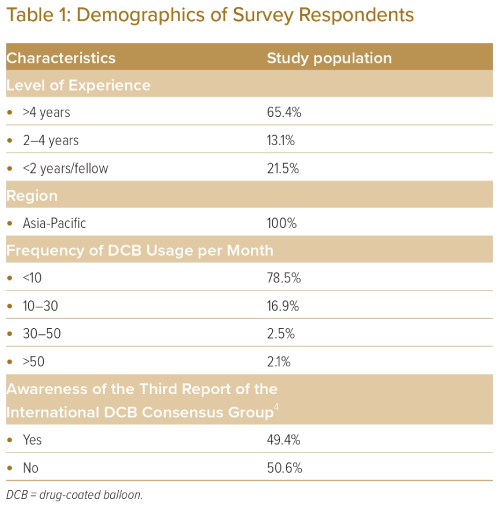
The baseline characteristics of the study population are listed in Table 1. The total number of respondents was 237. A total of 31.6% of respondents were from Singapore, 21.8% were from China, 17.6% were from Japan, 15.0% were from the Philippines and 14.0% were from Malaysia. A total of 65.4% of respondents had more than 4 years of experience as an interventional cardiologist, 13.1% had between 2 and 4 years of experience, and 21.5% had less than 2 years of experience. Slightly more than half (50.6%) of the respondents were unaware of the DCB expert consensus paper.
A total of 74.3% of respondents will actively consider using DCB for their patients undergoing PCI. However, most respondents (78.5%) use fewer than 10 DCBs per month, indicating low adoption rates of DCB strategy in practice. Further analysis showed that awareness of the DCB consensus paper had a positive correlation with operators actively considering using DCB for their patients (p<0.05), while years of experience and frequency of DCB usage did not.
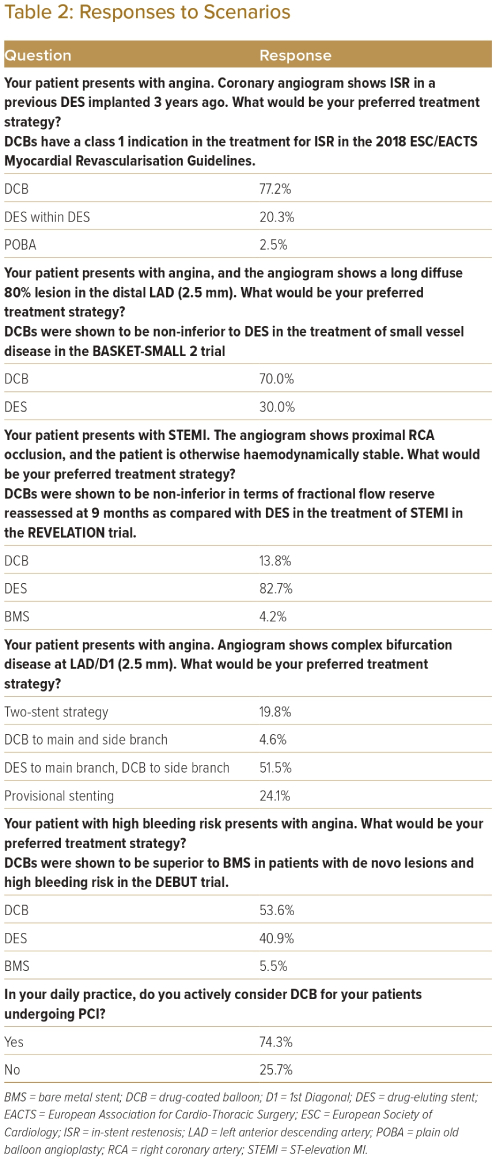
Table 2 lists the options that respondents would choose when faced with different scenarios during PCI. When treating a patient with in-stent restenosis (ISR), 77.2% of respondents would choose to deploy a DCB as compared with deploying a DES (20.3%) or using plain old balloon angioplasty (POBA) only (2.5%). A total of 70.0% of respondents would choose to deploy a DCB compared with DES when treating lesions in small vessels (defined as vessel diameter <2.5 mm). For patients with high bleeding risk, 53.6% of respondents would choose to deploy a DCB compared with 40.9% who would deploy a DES and 5.4% who would use POBA only. When faced with bifurcation disease, 51.5% of respondents would choose to deploy a DES in the main branch and DCB in the side branch, 24.1% would choose to deploy DES only in the main branch, 19.8% would choose a 2-stent strategy, and 4.6% would choose to deploy DCB both in the main and the side branch. When treating patients with ST-elevation MI (STEMI), most interventionists would still choose to deploy a DES (82.7%) as compared with a DCB (13.8%) or a bare metal stent (4.2%).
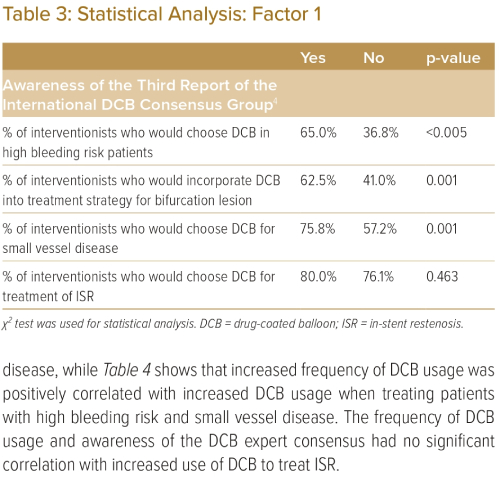
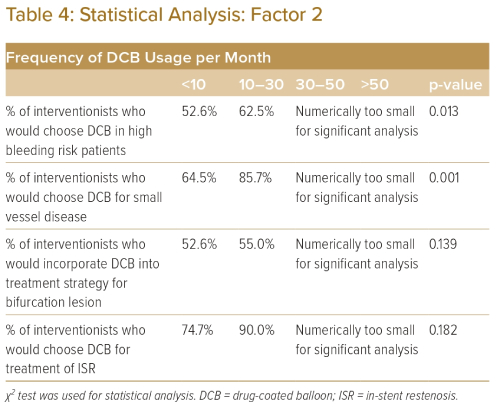
Table 3 demonstrates that awareness of the DCB expert consensus paper had a positive correlation with increased DCB usage when treating patients with high bleeding risk, bifurcation disease and small vessel disease, while Table 4 shows that increased frequency of DCB usage was positively correlated with increased DCB usage when treating patients with high bleeding risk and small vessel disease. The frequency of DCB usage and awareness of the DCB expert consensus had no significant correlation with increased use of DCB to treat ISR.
Discussion
DCB deployment has previously been shown to be a viable strategy during PCI. In a meta-analysis with more than 1,500 patients, the DCB-only strategy was shown to have good outcomes with no acute or subacute thrombosis.5 Based on the 2018 ESC/EACTS (European Society of Cardiology/European Association for Cardio-Thoracic Surgery) myocardial revascularisation guidelines, DCBs have a class 1 indication in the treatment of ISR.6 As more data become available, the indications for DCB could increase.
The BASKET-SMALL 2 study evaluated the use of DCB in small vessel disease.7 It demonstrated non-inferiority regarding MACE compared with second-generation DES at 12 and 36 months. These results were also consistent with other randomised controlled trials.8,9 More recently, the PICCOLETO 2 trial comparing DCB with second-generation DES in small vessel disease showed lesser late lumen loss with DCBs and no difference in MACE at 12 months.10 Previous studies have also shown low rates of restenosis at up to 3 years with DCB deployment.3
The use of DCBs may become more common as increasing numbers of patients present with bleeding risk, particularly given that the volume of PCI in elderly patients is expected to increase as the average lifespan of patients increases. This is especially pertinent in East Asian patients, who have a higher predisposition to bleeding risks compared with the western population.11 DCBs can potentially allow for a shorter duration of dual antiplatelet therapy (DAPT), which in turn could reduce bleeding complications caused by DAPT, or stent complications due to abrupt discontinuation of antiplatelet therapy due to other reasons.12 While the recent Master-DAPT trial showed clinical outcomes favouring short-term DAPT, 73.8% of the study population had PCI performed in a single vessel (73.8%).13 Only 5.7% of the study population had PCIs that involved stenting into the left main coronary artery, suggesting that the data may not be generalisable to patients who had undergone complex/left main PCI. As such, DCBs may still have a place in this scenario. Moreover, DCBs have also been demonstrated to be effective in side branch PCI as part of the treatment strategy for complex bifurcation lesions, thereby potentially avoiding two-stent strategies, which can carry a higher risk of stent thrombosis and may require a longer duration of DAPT.14,15 A longer duration of DAPT increases the risk of bleeding, which is associated with increased adverse outcomes such as mortality, non-fatal MI and repeat revascularisation. This makes DCB particularly attractive when treating patients at high bleeding risk, which is, unfortunately, becoming more prevalent. Our results show that slightly more than half of the respondents would choose to use DCBs in patients with high bleeding risk. This may increase as data emerge from ongoing randomised control trials such as the DCB-HBR trial.
With regard to the management of STEMI, most operators surveyed would still opt for DES over DCB, which was an expected result given that current guidelines recommend DES implantation as the first-line treatment for STEMI. However, this is a paradigm that could be challenged in the future. The REVELATION trial studied the mean fractional flow reserve after treatment of STEMI with DCB or DES and did not show any statistical difference at 9 months.16 This was followed up by a retrospective study that showed that DCB was non-inferior to DES in the treatment of STEMI in terms of all-cause mortality.17 The EROSION study showed that the majority of patients presenting with the acute coronary syndrome (ACS) secondary to plaque erosion remained free of major adverse cardiovascular events without any stent implantation.18 Another study showed that stent implantation in plaque erosion might also affect vascular healing.19 These studies highlight another potential area for greater DCB usage. This is especially pertinent given that plaque erosion is the primary pathology in one out of four patients with ACS.
While more data are required before we can ascertain whether DCB is truly non-inferior or even superior to DES in terms of traditional hard outcomes such as mortality or MACE, these results are hypothesis-generating and will hopefully encourage further randomised control trials in these areas.
Despite the large volume of literature on DCB usage, the usage of DCB remains low, with most respondents using fewer than 10 DCBs per month. We postulate that this may be due to varying inclusion in major guidelines, given that DCBs are still not approved by the FDA (Food and Drug Administration) in the treatment of coronary lesions, leading to its exclusion from the American College of Cardiology/American Heart Association guidelines. This is in contrast to the ESC guidelines, which have incorporated DCBs for a number of years. However, this could change in the near future with newer trials in the US evaluating the safety and efficacy of DCBs in coronary artery disease, such as the AGENT IDE trial.
Most of the respondents in this study were unaware of the DCB expert consensus paper. The DCB expert consensus paper succinctly summarises the potential advantages of DCB, and current and potential indications, providing valuable guidance in DCB usage in PCI today. This could encourage operators to use more DCBs, given the positive correlation between awareness of the DCB consensus paper and the usage of DCBs in practice seen in the present study. Operators who had used DCBs more frequently in their practice were also more likely to use DCBs in various scenarios. We postulate that the increased experience was likely to have enabled operators to become more comfortable with DCBs, given that they would have better understood the technical aspects of DCB delivery and deployment and better appreciated the potential indications for DCBs.
Although >50% of operators were unaware of the DCB expert consensus paper, most operators would still use DCBs when treating small vessel disease, ISR, bifurcation disease or in patients with high bleeding risk. Most of the available literature on DCBs is focused on these three areas, which may have explained this finding. We believe that with greater awareness of the DCB expert consensus paper and the emerging data on DCB usage, DCBs may become more ubiquitous during PCI.
Just as stents have moved on from eluting paclitaxel to eluting mammalian target of rapamycin (mTOR) inhibitors, such as sirolimus and everolimus, the newer generation of DCBs coated with -limus agents have started to enter the market. Large-scale international randomised control trials evaluating these newer generation DCBs against DES, such as the TRANSFORM II and PICCOLETO III, are already under way. We look forward to these results to further guide us and expand on the usage of DCBs in PCI.
There are some limitations to our study. First, the sample size of 237 is modest. Second, the survey distribution method may mean that not all groups of operators are well represented. Hence, the results captured in this survey may not be fully representative of operators in the Asia-Pacific region. Moreover, other important factors may have affected DCB usage, such as DCB availability, PCI volume or financial costs, which were not factored into the survey. Data on reimbursement of DCB costs in the different countries, which would intuitively be factored into the PCI strategy, were also unavailable. While we tried to include lesions of different complexity in the scenarios posed to the respondents, the numerous permutations make it nearly impossible for this factor to be adequately represented and studied in a survey that was intentionally kept short and succinct to improve response rates. Third, data on the total number of PCIs performed by each operator were unavailable, which would have better represented the frequency of DCB usage statistically.
Conclusion
The DCB expert consensus paper can improve awareness of the potential uses and of the literature behind DCBs. As operators become more experienced with DCBs, the usage will likely become more commonplace during PCI. There is already ample published literature establishing the safety and efficacy of DCBs in specific scenarios. With time and more emerging literature evaluating the safety and clinical outcomes data of DCBs, the list of indications for DCBs could increase. Ultimately, DCBs may be a more straightforward solution as patient presentations become more complex. 
Clinical Perspective
- Drug-coated balloons (DCBs) represent an attractive method of revascularisation of coronary artery disease without a requirement for permanent implantation.
- According to operators in the Asia-Pacific region, scenarios in which DCBs are commonly used include in-stent restenosis, small vessel disease (<2.5 mm) and high bleeding risk.
- As more literature evaluating the safety and clinical outcomes data of DCBs becomes available, the list of indications for DCBs could increase.
- As our patients present with more complex coronary anatomies, DCBs may present a more straightforward solution.