Postoperative right ventricular failure is a major cause of postoperative mortality and morbidity after implantation of a left ventricular assist device (LVAD).1 The morphological right ventricle (RV) is more sensitive to afterload than the morphological left ventricle (LV). In patients with concordant atrioventricular and ventriculo-arterial connections in whom the morphological RV is the sub-pulmonic ventricle, sildenafil can be used to manage postoperative right ventricular failure after LVAD implantation because of a reduction in pulmonary vascular resistance (PVR).2
Sildenafil is administered in adults with congenitally corrected transposition of arteries (ccTGA) for the management of postoperative subpulmonic ventricular failure after LVAD implantation. Compared with the morphological RV, the morphological LV can adapt more successfully to afterload.
Patients with ccTGA have discordant atrioventricular and ventriculo-arterial connections; the subpulmonic ventricle is the morphological LV. Haemodynamic data on the usefulness of sildenafil on the subpulmonic morphological left ventricle (pLV) have not yet been reported. We report the haemodynamic effects and usefulness of sildenafil on the pLV in a patient with ccTGA after LVAD implantation.
Case Report
A 45-year-old man was diagnosed with ccTGA at birth. He had discordant atrioventricular and ventriculo-arterial connections; the subpulmonic ventricle was the morphological LV (the pLV), and the systemic ventricle was the morphological RV (i.e. sRV). The patient underwent DDD-type (dual chamber) permanent pacemaker implantation for complete atrioventricular block at the age of 33 years.
Aged 41 years, he was admitted to our hospital for decompensated heart failure. Echocardiography showed severe systemic atrioventricular tricuspid valve regurgitation (TR), decreased ejection fraction to 25% of the sRV, dyssynchrony of the sRV and preserved systolic function of the pLV. He underwent tricuspid valve repair, pulmonary artery banding (PAB) and cardiac resynchronisation therapy.
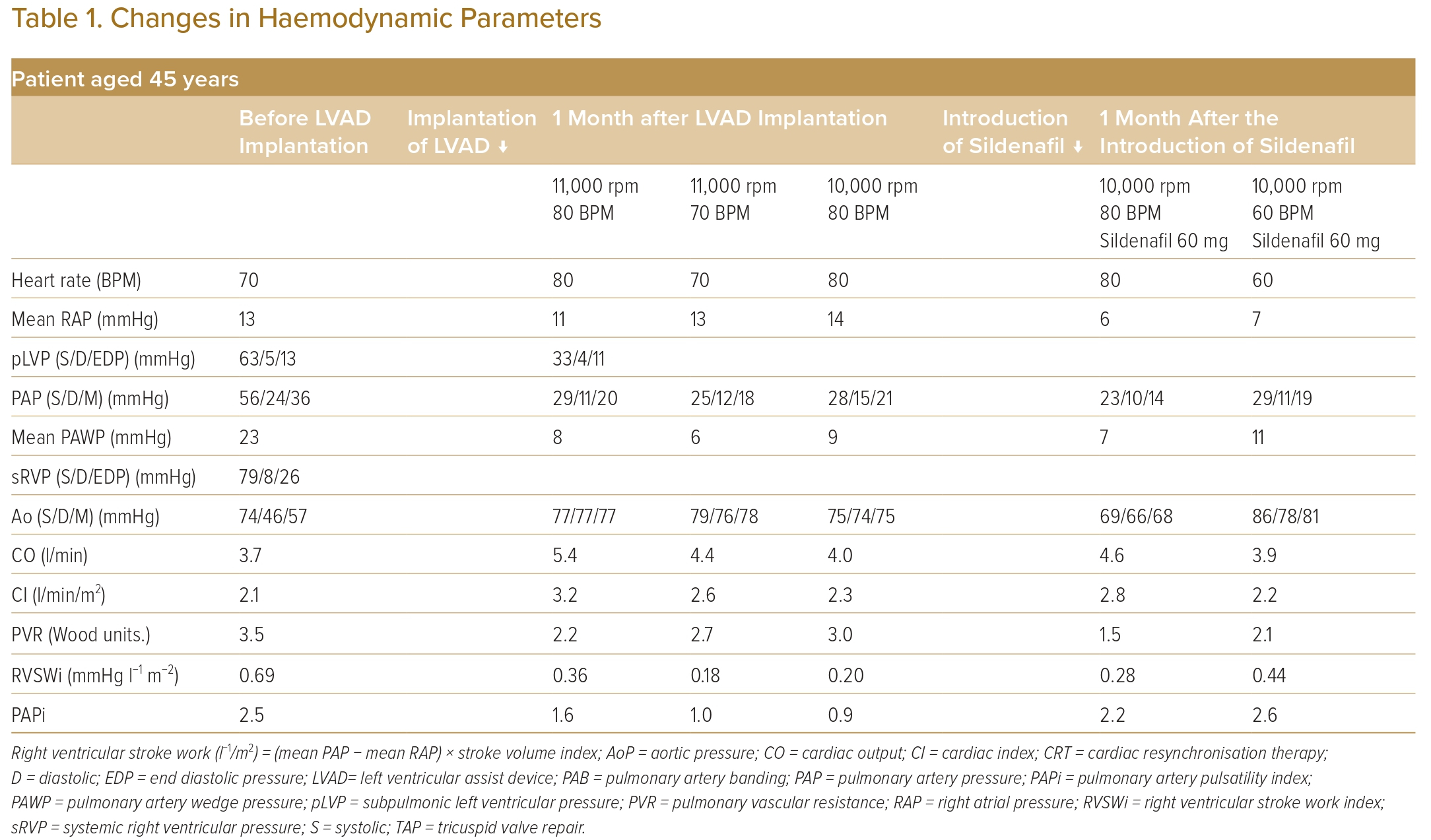
However, at the age of 45 years, he was hospitalised again for decompensated heart failure with progressive TR. Owing to the progressive haemodynamic parameters (pulmonary artery wedge pressure of 23 mmHg, systolic pLV pressure of 63 mmHg, systolic sRV pressure of 79 mmHg, and PAB pressure gradient of 15 mmHg) and severely impaired exercise tolerance (peak VO2 11 ml/kg/min), LVAD implantation was indicated (Table 1).
During LVAD implantation, the pulmonary artery band was removed, and a Jarvik 2000 (Jarvik Heart) was implanted in the apex of the sRV. The rotation speed of the LVAD was set to 11,000 rpm, and the pacing rate was set to 80 BPM.
After the surgery, the right atrial pressure reduced from 13 mmHg to 11 mmHg, and the cardiac index increased from 2.1 l/min/m2 to 3.2 l/min/m2.
Anticoagulation therapy for the LVAD was started; therefore, invasive procedures, such as exchanging the cardiac resynchronisation therapy generator, were avoided. To save the cardiac resynchronisation therapy battery, the pacing rate was decreased.
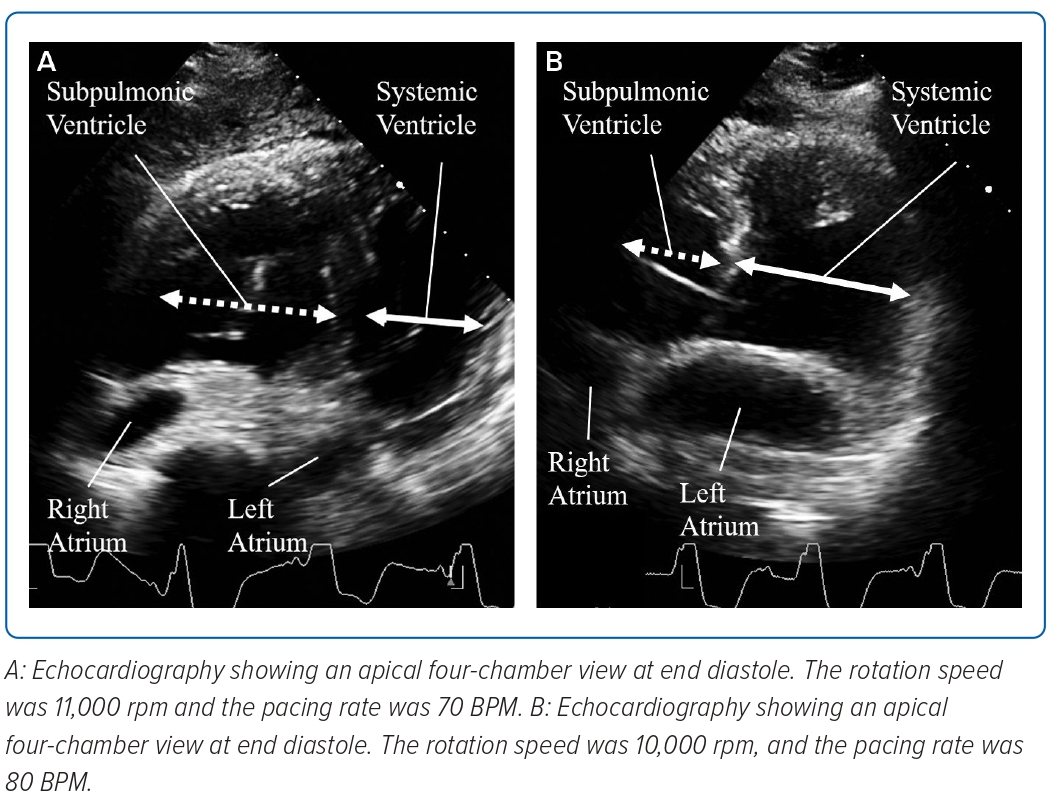
When the pacing rate decreased to 70 BPM, pLV dilatation and a shift of the ventricular septum to the left side were observed in diastole (Figure 1A). Right atrial pressure/pulmonary artery wedge pressure ratio increased to 2:1, the pulmonary artery pulsatility index (PAPi) was 1.0 and the right ventricular stroke work index (RVSWi) exacerbated to 0.18 mmHg l−1/m2 (Table 1).
The rotation speed of the LVAD was decreased to 10,000 RPM and the pacing rate was restored to 80 BPM. Consequently, the pLV dilatation and ventricular septum shift disappeared (Figure 1B).
Regarding pLV management, sildenafil 60 mg daily was started. PVR then decreased from 3.0 to 1.5 Wood units. Four months later, the haemodynamic parameters of the pLV, namely right atrial pressure/pulmonary artery wedge pressure ratios, PAPi and RVSWi, improved. Although the pacing rate decreased to 60 BPM again, the haemodynamic parameters did not worsen and pLV dilatation and ventricular septum shift did not reappear (Table 1).
Discussion
This case study examines the haemodynamic effects of sildenafil on the pLV based on haemodynamic parameters. Right atrial pressure/pulmonary artery wedge pressure ratio, PAPi and RVSWi are known haemodynamic indicators of the morphological RV as the subpulmonic ventricle and predictors for postoperative subpulmonic ventricular failure after LVAD implantation.3
Before LVAD implantation, PAPi was preserved in this case at 2.5, which is comparable with PAPi previously reported in patients with subpulmonic ventricular failure.4 However, the cutoff values of these parameters for evaluating morphological left ventricle function as the subpulmonic ventricle are unknown.
When the pacing rate decreased to 70 BPM after LVAD implantation, the right atrial pressure/pulmonary artery wedge pressure ratio increased to 2:1 from 1:3, PAPi decreased to 1.0 from 1.6, and RVSWi reduced to 0.18 mmHg l−1/m2 from 0.36 mmHg l−1/m2.
Several reasons were considered for the shifts in haemodynamic parameters. First, structural changes in the ventricles impair the subpulmonic ventricular function. Volume overload of the subpulmonic ventricle and acute unloading of the systemic ventricle by the LVAD can lead the ventricular septum to shift the left side. This twist of the pericardium and ventricles decrease septal function and reduce the contribution of the systemic ventricle to the contractile function of the subpulmonic ventricle. Usually, most subpulmonic ventricular contraction is contributed by systemic ventricular contractility; hence, this structural change significantly reduces subpulmonic ventricular contractility.5
In the present case, when the pacing rate fell to 70 BPM after LVAD implantation, the ventricular septum shifted to the left side towards the systemic RV, and the haemodynamic parameters of the subpulmonic ventricle were reduced.
The Jarvik 2000 is a continuous-flow LVAD; thus, systemic circulation was more dependent on the rotation speed of the LVAD than on the pacing rate. In contrast, pulmonary circulation was mainly dependent on the pacing rate. When the pacing rate decreased, pulmonary circulation from the pLV weakened; however, the systemic circulation continuously flowed into the pLV. This imbalance between the pulmonary and systemic circulation leads to pLV dilatation and a shift of the ventricular septum to the left side. These changes induce acute pLV dysfunction.
Second, pLV diastolic dysfunction is caused by PAB. PAB was performed as a palliative procedure to improve sRV function and TR.6 However, in older patients, PAB causes pLV hypertrophy and fibrosis, which lead to the progression of ventricular diastolic dysfunction.7 Ventricular diastolic dysfunction leads to poor tolerance of the preload and easily shifts the ventricular septum to the left side under LVAD circulation.
The administration of pulmonary vasodilators is a known medical management strategy for subpulmonic morphologic right ventricular failure after LVAD implantation. According to Imamura et al., postoperative subpulmonic morphologic right ventricular failure after LVAD implantation can be successfully managed with sildenafil.2
Pulmonary vasodilators decrease PVR and improve pulmonary circulation. Similar effects on the pLV have been observed in patients with ccTGA and pulmonary artery hypertension.8,9 However, PVR is low after LVAD implantation because patients with a high PVR (>5 Wood units) are excluded as candidates for heart transplantation and LVAD implantation. The haemodynamic effects of sildenafil on the pLV with low PVR have not been reported.
In the present case, PVR decreased to 1.5 Wood units from 3.0 Wood units after sildenafil administration. Additionally, the haemodynamic parameters of the subpulmonic ventricle improved: the right atrial pressure/pulmonary artery wedge pressure ratios decreased to 0.8 from 1.5; PAPi increased to 2.2 from 0.9; and RVSWi increased to 0.28 from 0.20 mmHg l−1/m2.
The improvement in PAPi has clinical significance for the management of LVAD circulation. Morine et al. reported the haemodynamic characteristics for patients with postoperative subpulmonic morphologic right ventricular failure after LVAD implantation. After LVAD implantation, PAPi usually decreases temporarily and gradually recovers. However, some patients have residually impaired PAPi, which induces postoperative subpulmonic morphologic right ventricular failure.10
Sildenafil is thought to have limited haemodynamic effects on the pLV compared to subpulmonic morphological RV and pulmonary hypertension. However, this report showed sildenafil had clinically significant effects on the pLV. Sildenafil has limited effects on pLV afterload; however, the improved pulmonary circulation adjusts the balance between the pulmonary and systemic circulation. This prevents the abnormal ventricular septum shift, which leads to preserved septal function and maintains the contribution of the sRV to the contractile function of the pLV. These combined effects may improve the haemodynamic function of the pLV.
Conclusion
Sildenafil demonstrated haemodynamic effects and usefulness on subpulmonic morphological left ventricle in patients with ccTGA after LVAD implantation. 
Clinical Perspective
- In patients with a subpulmonic morphological right ventricle, sildenafil can be used to manage postoperative subpulmonic ventricular failure after left ventricular assist device (LVAD) implantation.
- However, data regarding the haemodynamic effects on the subpulmonic morphological left ventricle are lacking.
- This case study describes the haemodynamic effects of sildenafil on the subpulmonic morphological left ventricle by using the haemodynamic parameters of intracardiac pressure and ultrasound cardiography.
- This report might assist in the management of LVAD circulation and prevention of subpulmonic ventricular failure in patients with subpulmonic morphological left ventricle.