Congenital complete heart block (CCHB) is a severe life-threatening rare disorder with an incidence of 1 in 15,000–20,000 live births.1 The mortality rate reaches 45% in the first two decades of life when CCHB is diagnosed in utero.2 Implantation of a permanent pacemaker (PPM) is recommended for symptomatic CCHB patients with syncope, congestive heart failure or chronotropic incompetence and for asymptomatic CCHB patients with high-risk features.1 General expert consensus states that epicardial pacemakers have been applied in younger patients. However, long-term pacing is usually required for patients diagnosed with CCHB.
Physiological pacing, such as His bundle pacing (HBP) and left bundle branch pacing (LBBP), has been developed to overcome the negative side-effects of right ventricular (RV) pacing as it activates the normal cardiac conduction system.3,4 Disadvantages of HBP include a narrow target area, a higher and unstable pacing threshold, lower R-wave amplitude, potential development of conduction block distal to pacing site, early battery depletion and risk of lead revision due to dislodgement.3–6
Huang et al. developed a transseptal approach for LBBP, which offers a larger target area, good lead stability, low pacing threshold, large R-wave and lower probability of distal conduction block development.6,7 Thus, LBBP has been recognised as the alternative to HBP as it overcomes its many limitations. Until recently, LBBP in children under 5 years has not been reported.
Case Report
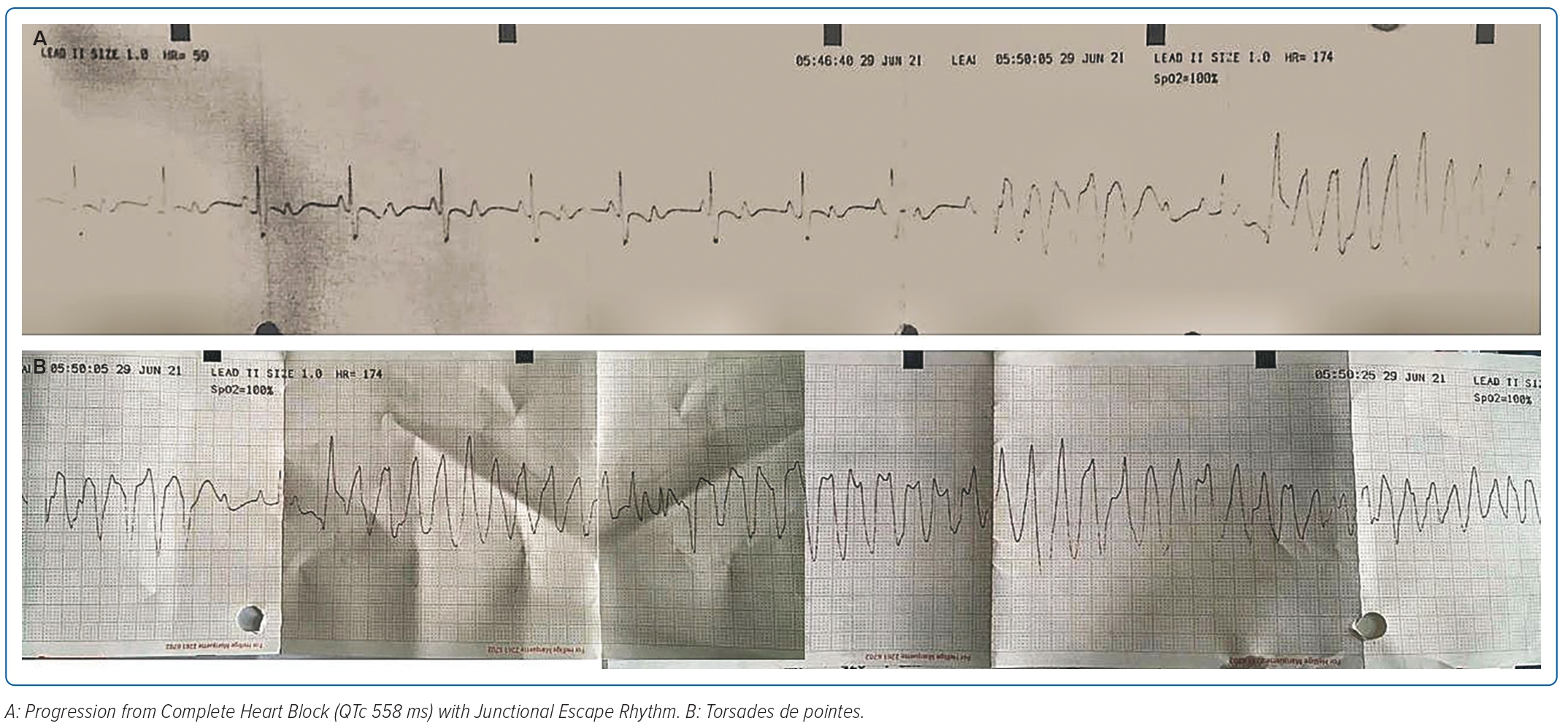
A 2-year-old girl weighing 12 kg was referred to the emergency room of Harapan Kita National Heart Center, Jakarta, Indonesia, from the National Brain Center Hospital in Jakarta due to the return of spontaneous circulation after cardiac arrest, bradyarrhythmia and repeated seizures with loss of consciousness. The seizure appeared 3 days before and worsened 3 hours prior to admission. She had a history of seizures; however, an EEG test and head CT scan conducted in 2020, when the patient was 1 year old, were normal. An epilepsy drug was administered due to recurrent seizures. Figure 1 shows an ECG during one epileptic episode that started from complete heart block with junctional escape rhythm of 65 BPM, corrected QT interval 558 ms (Hodges formula), which then progressed to torsades de pointes. Echocardiography showed left atrium and left ventricle (LV) dilatation with LV systolic ejection fraction (EF) of 45%, good right ventricular (RV) contractility and tricuspid annular plane systolic excursion of 15 mm. Laboratory examinations showed a potassium level of 3.6 mmol/l and N-terminal pro B-type natriuretic peptide (NT-proBNP) of 15,722 pg/ml (normal range <125 pg/ml). A temporary transvenous pacemaker was inserted via the right femoral vein and single-chamber PPM implantation was performed a few days later.
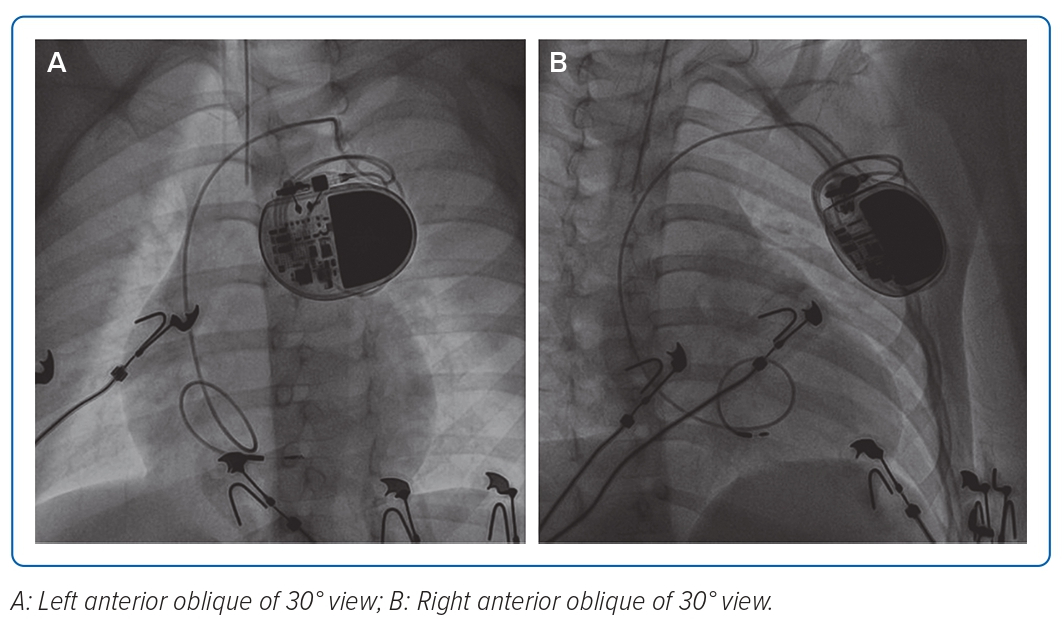
PPM implantation was performed under general anaesthesia. After obtaining left subclavian venous puncture, an 4.1 Fr 3,830–69 cm SelectSecure lead (Medtronic) through C315 His Sheath (Medtronic) was used to map the His bundle area assisted by the electrophysiology system. However, no His bundle potentials were recorded and no narrow QRS was achieved during high output pacing. Subsequently, the lead was moved at the ventricular side around 1–2 cm across the tricuspid valve and screwed for six turns into the interventricular septum with the help of fluoroscopy guidance (Figure 2). No LBB potential was recorded. Pace-mapping technique could not be performed because there were no documented intrinsic QRS complexes. The LBB area mapping was guided using fluoroscopy.
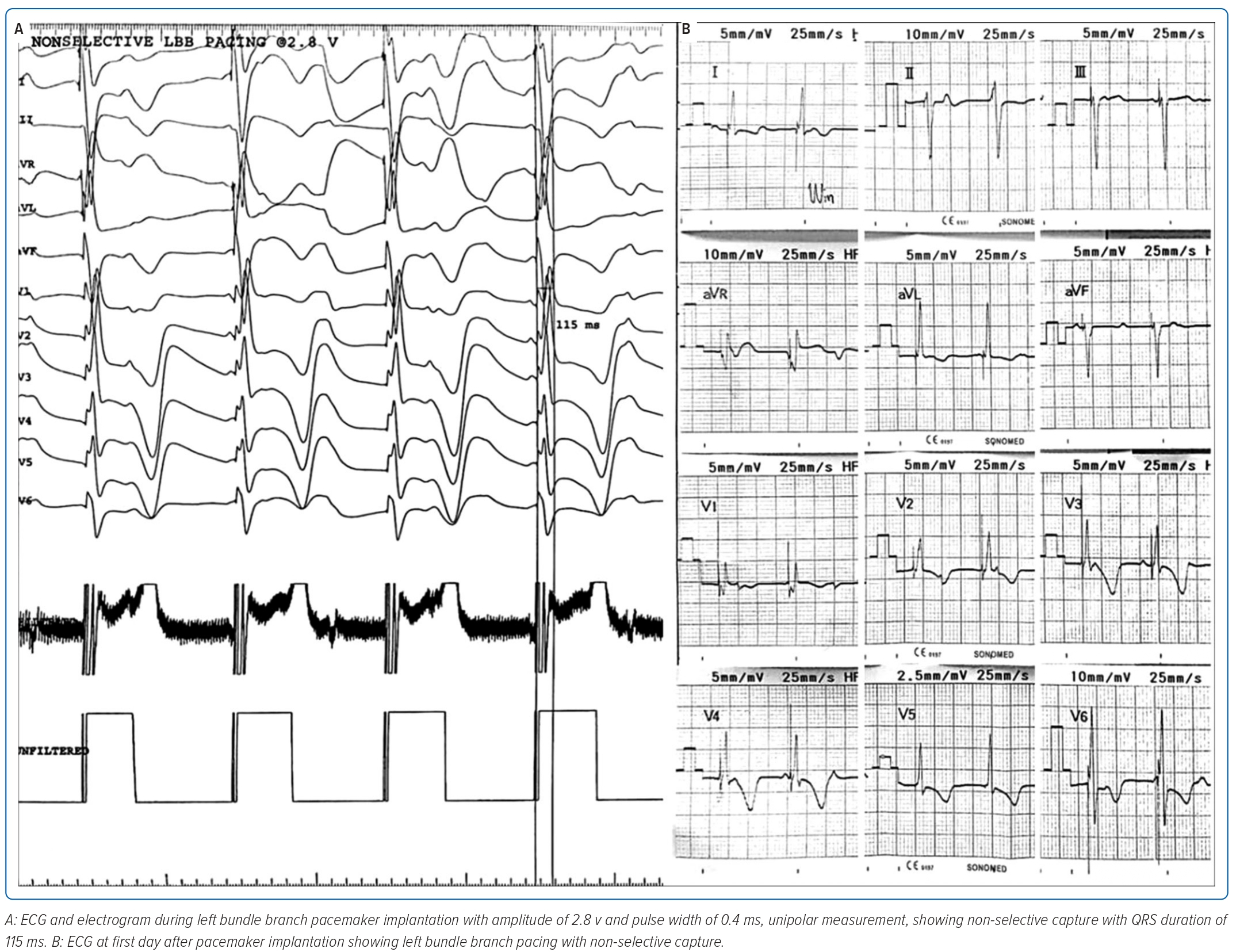
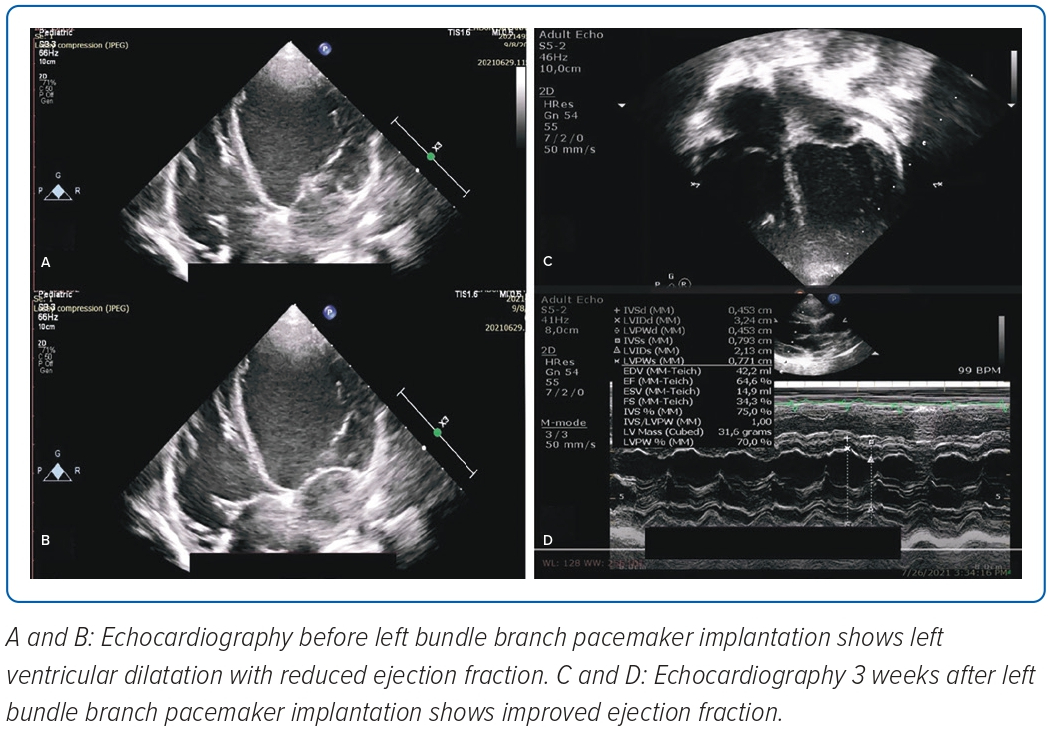
Non-selective LBB capture with right bundle branch block (RBBB)-paced morphology was achieved with QRS duration of 115 ms and in LV activation time of 50 ms with threshold 0.5V at pulse width 0.4 ms and impedance 893 Ω at 5.0 V and current 1.0 mA (Figure 3A). Figure 3B shows an ECG performed during the first day after PPM implantation that demonstrated ventricular pacing with non-selective LBBP capture. During 3 weeks follow-up, there were no recurrent seizures and echocardiography revealed an LVEF of 64%, good RV contractility and a tricuspid annular plane systolic excursion of 17 mm (Figure 4).
Discussion
PPM has been the mainstay of management for patients with CCHB. Previous guidelines state that PPM implantation is indicated for CCHB with wide QRS escape rhythm, complex ventricular ectopy or ventricular dysfunction. PPM implantation is also indicated for CCHB in infants with ventricular rate <55 BPM or with congenital heart disease and a ventricular rate <70 BPM. PPM implantation is reasonable for CCHB beyond the first year of life with an average heart rate <50 BPM or associated with symptoms related to chronotropic incompetence.1 Since CCHB patients require ventricular pacing more than 40% of the time, a pacing method that maintains physiological ventricular activation, such as cardiac resynchronisation therapy (CRT) or HBP, is preferred over RV pacing.8
LBBP has emerged as an alternative method to deliver physiological pacing, which can preserve LV synchrony by directly stimulating the normal cardiac conduction system. LBB provides a larger target for pacing as LBB comprises a wider area compared to His bundle; hence, there is a higher success rate.6 Aside from that, LBBP offers better lead stability since the lead is placed deep within the interventricular septum. Huang et al. reported favourable outcomes from choosing LBBP in heart failure patients with LBBB in which LVEF increased to 62% from baseline 32%, LV volume decreased, and New York Heart Association (NYHA) functional class improved from baseline IV to I within 1 year of PPM implantation.7
Another study enrolling 11 heart failure patients with LBBB also reported excellent results with significant improvement of LVEF and NYHA functional class.9 However, studies regarding the use of LBBP in children with CCHB are still quite limited. The first study of LBBP in a child with second-degree AV block and LBBB demonstrated outstanding preliminary results with LVEF increasing from baseline 49% to 61% and NYHA functional class improving from II to I within 3 months.10 Due to favourable outcomes from previous studies, LBBP is considered a suitable alternative to CRT and HBP.
Our patient had symptomatic CCHB presenting with recurrent seizures due to torsades de pointes caused by acquired prolonged QT interval related to epilepsy drug administration. The LVEF improved significantly after the LBBP implantation as a bail-out treatment. Mapping in the LBB area showed no LBB potential.
Similar to HBP, LBBP can also be selective and non-selective.5 With selective LBBP, there is a distinct isoelectric segment before the onset of paced QRS complex or paced QRS morphology identical to the intrinsic QRS complex.5,11 With non-selective LBBP, there is no isoelectric segment before the onset of the paced QRS complex, which indicates direct activation of the adjacent local myocardium and LBB.5,6 Non-selective LBBP is related to the proximity to conducting His bundle tissue, in which the pacing lead captures the His bundle and local myocardium but is close enough that the paced wavefront engages the His-Purkinje system relatively quickly, creating a pseudo-delta wave. Aside from that, paced QRS morphology is wider than the intrinsic QRS complex. Both selective and non-selective LBBP likely have overlapping mechanisms and the concept of a virtual electrode polarisation effect plays a role in creating fields of depolarisation in or around the areas of block.11 The patient responded well to LBBP. Our case also demonstrated similar results to previous reports. During 3 weeks follow-up, there were no recurrent seizures after PPM implantation and LVEF improved from 45% to 64%. Our case showed promising results regarding the use of LBBP in a CCHB patient. With further investigation from randomised controlled trials regarding long-term efficacy and safety of LBBP, it might become the definitive treatment for CCHB patients who require long-term pacing.
Conclusion
To the best of our knowledge, this case reports on the youngest patient to have LBBP implantation. During the follow-up period, the patient responded well to the LBBP with no more seizure episodes or increase in LVEF. Our study showed a promising result regarding the use of LBBP in children. However, further research is needed to analyse the long-term outcomes in the paediatric population. 
Clinical Perspective
- Left bundle block pacing (LBBP) has emerged as an alternative to His bundle pacing in delivering physiological pacing; this was especially useful in our case since the left ventricle ejection fraction was decreased.
- The implantation of LBBP in children under 5 years old has not been reported previously; however, in this case, we have seen a promising result.
- With further studies and randomised controlled trials, LBBP might become the definitive treatment, especially in paediatrics with patients with congenital complete heart block who require long-term pacing.