Staphylococcus aureus is a Gram-positive bacteria that normally colonises the skin and mucous membranes. Multiple skin punctures and the presence of vascular access catheters common among patients undergoing haemodialysis, compounded by an immunocompromised state due to their general medical condition, increase the likelihood of bloodstream contamination leading to bacteraemia and infective endocarditis (IE). In rare scenarios, the aorta may also be inoculated and cause degeneration of the intimal and medial layers, creating a mycotic aneurysm.1 Among the other causative organisms of mycotic aortic aneurysm (MAA) reported from the Philippines are Salmonella, Mycobacterium tuberculosis, Burkholderia pseudomallei and Neisseria gonorrhoeae.2–6 The difficulty in diagnosis and management is due to the rarity of the condition, the insidiousness of the presentation and the high-risk condition of the affected patients, all of which complicate medical and surgical treatment.
This case study describes a virulent methicillin-resistant S aureus (MRSA) IE complicated by contained rupture of MAA and post-procedural endoleak type IIIa, treated with culture-guided antibiotics and a timely hybrid, two-staged procedure combined with the deployment of two overlapped thoracic endografts.
A 75-year-old Filipino woman was admitted for fever, cough and back pain. She had stage II hypertension, type 2 diabetes and chronic kidney disease, and had been on maintenance haemodialysis since 2012. She underwent perm catheter insertion 5 months before admission due to a failed arterial-venous (AV) fistula creation and internal jugular vein catheter-related bloodstream infection. On physical examination, she was awake, in mild cardiorespiratory distress and had the following vital signs: blood pressure 140/80, heart rate 72 BPM, respiratory rate 21 breaths per minute, temperature 37.8°C, 02 saturation 97% at 1–2 l/minute via nasal cannula. There were bibasilar rales and decreased breath sounds on the left base. Her cardiac examination was unremarkable. Complete blood count showed anaemia (haemoglobin 79 g/dl, hematocrit 26%) and severe leukocytosis with a neutrophilic predominance (white blood cell count 23.3, neutrophils 87%, lymphocytes 3%). A chest X-ray revealed hazy densities in the left lower lung, suggestive of pneumonia with consolidation. Upon admission, she underwent a blood transfusion and was started on ceftriaxone 2 g IV every 24 hours and azithromycin 500 mg tablet every 24 hours. Blood cultures showed growth for Gram-positive cocci in clusters, which was identified at the 24th hour as MRSA. Antibiotics were changed to piperacillin–tazobactam and vancomycin following end-stage renal disease dosing.
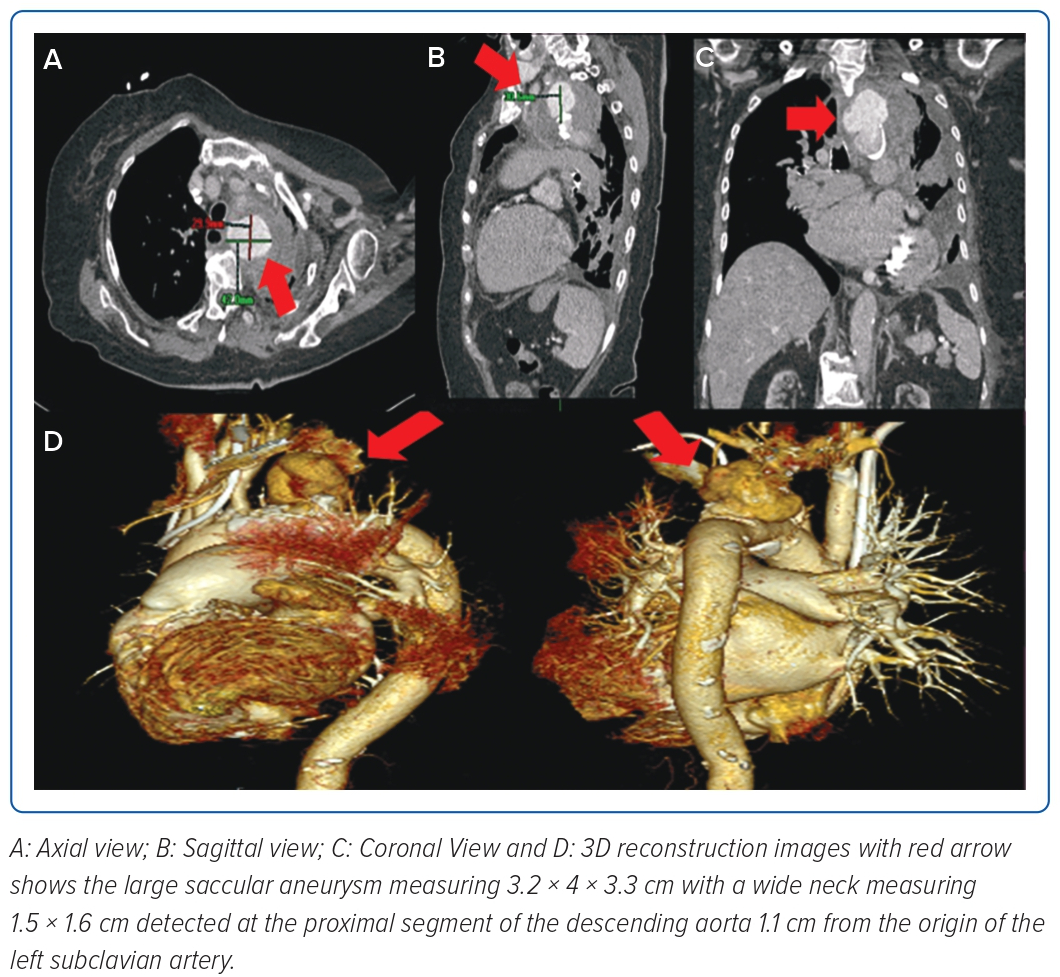
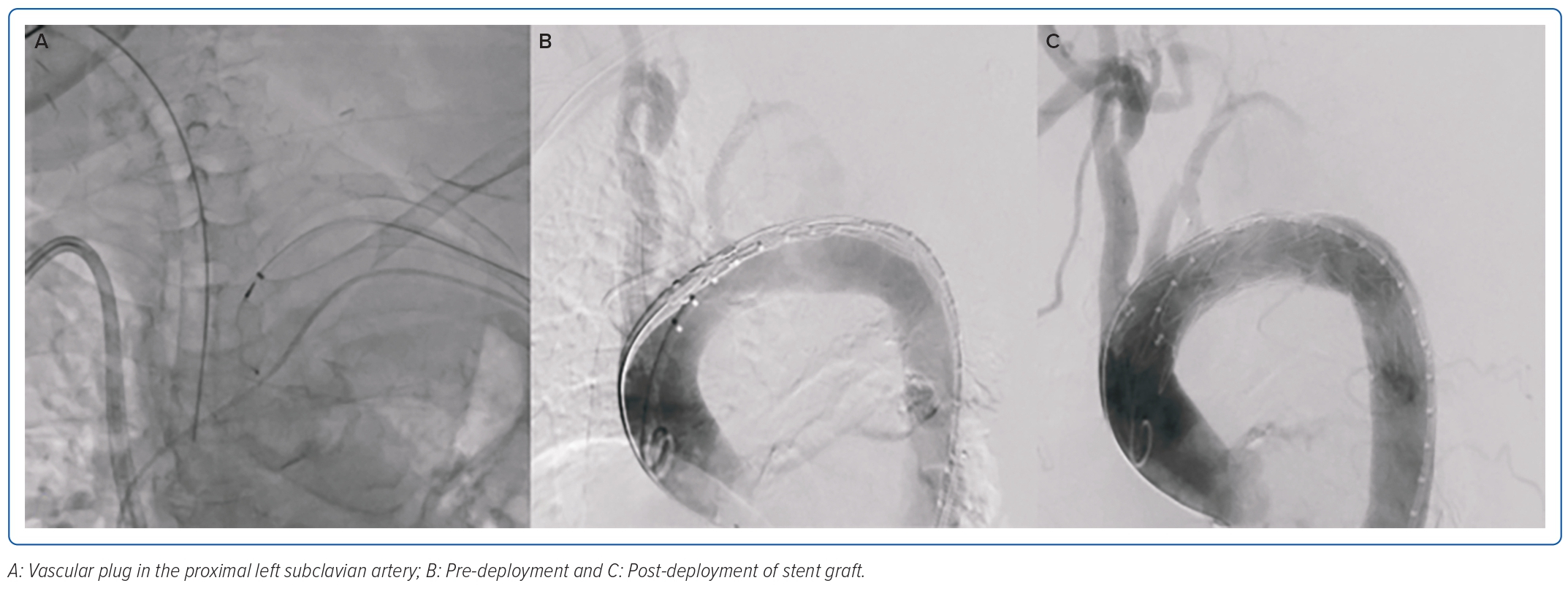
Fever resolved by the fifth day of hospitalisation, but leukocytosis persisted, leading to further investigation for potential additional sources of infection. The patient underwent a transoesophageal echocardiogram which showed a 1.1 cm oscillating linear echo density on the antero-middle segment of the anterior mitral valve on the ventricular side, with associated moderate mitral regurgitation. Antibiotics were then shifted to meropenem for pneumonia and vancomycin for IE. On the eighth hospital day, when her condition had not improved despite appropriate antibiotic therapy, a chest CT scan was done, which showed a wide neck saccular aneurysm of the aortic arch with contained rupture. On CT aortogram (Figure 1), the aneurysm was measured at 3.2 × 4 × 3.3 cm (craniocaudal × width × anterior–posterior) and neck at 1.5 × 1.6 cm (width × anterior–posterior) at the proximal segment of the descending aorta, 1.1 cm from the origin of the left subclavian artery. There was a heterogeneous fluid collection with evidence of contrast extravasation. Due to her multiple comorbidities and frail condition, the medical team opted for a less invasive approach, which was a two-step procedure to be done after bacteraemia had been cleared. On the 18th day of hospitalisation, debranching using a left subclavian/left common carotid artery side-to-side anastomosis using a straight ringed polytetrafluoroethylene (PTFE) 8 mm graft, a left common carotid to right common carotid artery side-to-side anastomosis using a straight ringed PTFE 8 mm graft, and ligation of the left proximal common carotid artery was done successfully. Two days after debranching, occlusion of the left subclavian artery using an Amplatzer vascular plug sized 8 × 16 mm followed by thoracic endovascular aortic repair (TEVAR), using a self-expanding stent graft 32 mm × 83 cm positioned approximately 5 cm after the brachiocephalic artery and 40 cm after the left subclavian artery was deployed (Figure 2).
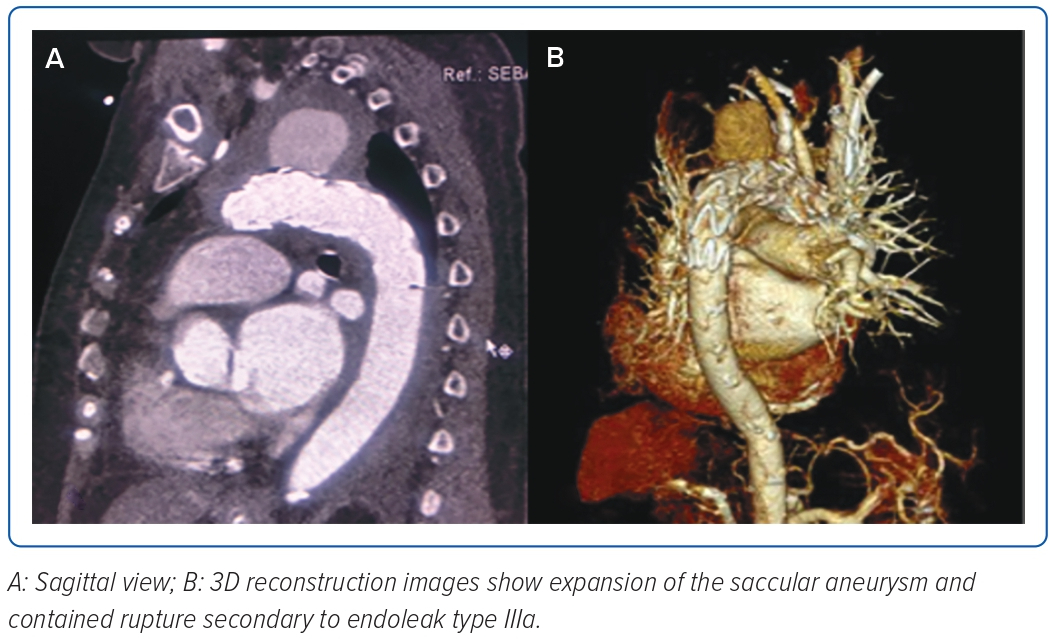
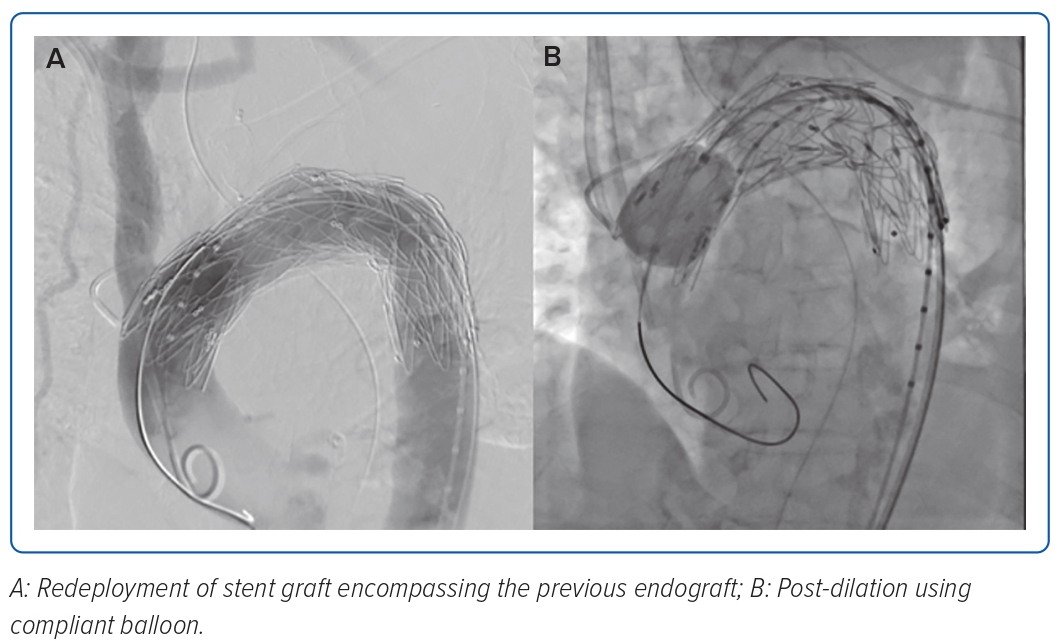
The completion angiogram demonstrated a correctly positioned stent graft, total exclusion of the aneurysm, minimal type IV endoleak, and a patent left common carotid. The patient’s condition stabilised; however, on the 14th day post-TEVAR, hypotension with decreasing haemoglobin ensued. A repeat CT aortogram showed expansion of the saccular aneurysm and contained rupture with possible endoleak, and a conventional aortogram (Figure 3) with selective injection of gutters demonstrated a type IIIa endoleak at the proximal endograft. She underwent endograft repair (Figure 4) using a 32 × 28 mm tapered stent graft deployed before the left common carotid artery overlapped from the previous endograft covering the endoleak, followed by post-dilatation with a compliant balloon. The completion angiogram demonstrated resolution of the previously noted endoleak. A repeat blood culture and transoesophageal echocardiogram showed the resolution of the bacteraemia and vegetation. The patient completed a 3-week course of meropenem and was discharged stable and improved with vancomycin as outpatient parenteral antibiotic therapy for 6 weeks and a total of 12 weeks duration. A repeat CT aortogram at discharge, 6 and 12 months after the procedure showed regression of the saccular aneurysm with no evidence of endoleaks.
Discussion
MAA is a potentially fatal infection where microbial inoculation of a diseased aortic endothelium during bacteraemia leads to degradation of the intimal and medial layers of the aortic wall, causing an aneurysm to form.2 Incidence may be as low as 0.6% in developed nations to as high as 13% in Taiwan.7 It may be secondary to contiguous infections such as osteomyelitis or intra-abdominal infections, bacteraemia seeding, direct bacterial inoculation after arterial injuries, or septic embolisation from endocarditis – the latter reportedly being the most common cause.8 In the case presented, both IE and bacteraemia could have contributed to the development of the aneurysm. Aside from the multiple sources of infection, immunosuppression due to end-stage renal disease, the patient’s other comorbidities, frequent vascular injuries from arteriovenous fistula access, and central line catheter insertions all played a significant part.8 Given the haematological spread, any arterial segment may be affected, but the most common areas are the intracerebral circulation and the suprarenal abdominal aorta and involvement of the thoracic aorta is uncommon.
The difficulty in managing MAA is partly due to the delay in diagnosis, given its non-specific symptomatology. The usual presenting symptom is fever, along with vague pains in the back, chest or abdomen, and blood tests revealing leukocytosis.9 Upon admission, the patient already had these symptoms, but they were initially attributed to pneumonia. The lack of clinical improvement despite adequate antibiotic therapy and persistent leukocytosis was what eventually led to the finding of the IE complicated by contained rupture of MAA. The danger with late diagnosis in these cases is the aneurysm’s propensity for complete rupture, reported to occur in 50–85% of cases, which may rapidly prove fatal.8 Therefore, a high index of suspicion is important, particularly for patients with risk factors and blood cultures that are positive for the common causative organisms, especially S aureus. Appropriate imaging can immediately reveal the diagnosis.
Treatment is two-pronged: antibiotics and surgery.10 Culture-guided antibiotic therapy should be started immediately, which would be vancomycin for MRSA, usually with a prolonged course of at least 6 weeks, given the presence of IE as stated in the guidelines.11,12 Pre-operative antibiotic therapy for 2–6 weeks is advised and ideally once the resolution of bacteraemia is achieved to avoid graft infection unless emergency surgery is indicated.13 Standard surgical repair involves aneurysm resection with tissue debridement and revascularisation through in situ reconstruction or extra-anatomical bypass.9 However, this procedure has in-hospital mortality rates as high as 30–40% and is unsuitable for high-risk patients with severe multiple comorbidities, poor anatomy or rupture. Since most patients with MAA are high risk, endovascular aortic repair (EVAR) of aneurysms has become a promising alternative and is recently gaining popularity. A successful case performed by Xodo et al. used multiple parallel grafts with chimney/periscope configuration combined with endograft deployment, which spared a patient with high surgical risk and a ruptured mycotic aneurysm from open surgery or debranching using EVAR.13 Mortality rates vary, but a review by Sorelius et al. shows 30–90-day mortality for MAAs of the descending aorta to be 15% for EVAR and 7–20% for open surgical repair.14 A European multicentre trial conducted by Sorelius et al. in 2014 showed EVAR to have good short-term outcomes, with 91% survival at 30 days, significant (19%) fatal infection-related complications often during the first postoperative year and a relatively good long-term outcome of 55% survival at 5 years. It also showed adverse long-term outcomes among non-Salmonella-positive blood cultures, specifically a higher risk of late death and fatal infection complications.15 A debranching procedure was necessary before the TEVAR, given the location of the aneurysm close to the aortic arch branches. This is to avoid compromising blood flow to the left carotid circulation by creating an alternative supply source before graft implantation.
Aside from mortality and infection-related complications often mentioned with EVAR, another possible complication as seen in this patient is an endoleak, which is a continued blood flow in the aneurysm sac outside the stent graft.16 This can be categorised into five types depending on the cause of the leak; a type II endoleak was prevented by deploying a vascular plug in the proximal left subclavian artery, while a type IIIa was discovered in this patient 2 weeks after the procedure presented with persistent anaemia and hypotension. This is caused by leakage between different parts of the stent graft, which may either be due to modular disconnection between the stent body and limb or disruption of the fabric of the stent graft. The incidence of this complication is rare; for type III endoleaks specifically, the EVAR1 trial, OVER trial, and the EUROSTAR registry cite its incidence at 3–4.5%.16 A retrospective study by Maleux et al. found the incidence to be at 2.1%, more often seen in first- and second-generation stents.16 Most of the patients were managed endovascularly, similar to the patient in this case report, with minimal complications. However, reintervention does not preclude the recurrence of the endoleak.
The increased risk for infection-related complications suggests an extension of antibiotic therapy for prolonged duration after successful endovascular MAA treatment.14 Since there is no widely accepted standard for the management of MAA, an individualised duration of antibiotic therapy is advocated.10 The team decided that the preoperative culture-guided antibiotics for 2 weeks administered before TEVAR, with the resolution of bacteraemia would be adequate to prevent graft infection in consideration of the urgency of the procedure. The additional 6 weeks of outpatient parenteral therapy was based on the patient’s condition and the results of close surveillance imaging. A CT aortogram at discharge, the 6- and 12-month follow-up showed regression of the saccular aneurysm with no complications. Hence, the antibiotic treatment was completed in a total of 12 weeks. A single postoperative antiplatelet therapy is also advised to prevent graft thrombosis and peripheral embolisation.17 Finally, addressing the initial cause, which was the repeated infections of the haemodialysis access, is also necessary to prevent reinfection.
Conclusion
AV fistula manipulations and catheter insertions are predisposing factors for staphylococcus infection. A saccular mycotic aneurysm with a contained rupture should be suspected in patients presenting with progressive leukocytosis, persistent anaemia and IE. A hybrid two-step procedure with debranching and anastomosis of aortic branches followed by transthoracic endovascular aortic repair may be employed. An endoleak type IIIa is a rare and fatal postoperative complication that can be addressed by the deployment of an endograft stent in an overlapped fashion covering the leak. Long-term management involves single antiplatelet therapy, antibiotics and close surveillance. 
Clinical Perspective
- Mycotic aortic aneurysm should be a differential diagnosis for immunocompromised patients with vascular access presenting with bacteraemia, infective endocarditis and persistent signs of infection despite antibiotic therapy.
- Debranching with thoracic endovascular aortic repair is a viable, less invasive option for mycotic thoracic aneurysm repair in high-risk patients.
- Management does not end with aneurysm repair, since prolonged culture-guided antibiotic therapy and close postoperative monitoring are also important.
- The duration of antibiotic therapy is based on good clinical judgement using ancillary tests and the clinical response of the patient.