Fractional flow reserve (FFR) using an invasive pressure wire has a Class 1A recommendation for guiding coronary revascularisation in stable coronary artery disease (CAD).1 A pressure wire-based index is used during coronary angiography to assess the potential of a coronary stenosis to induce myocardial ischaemia.2–5 There is robust data for deferring percutaneous coronary intervention (PCI) in lesions deemed not significant by FFR versus angiography alone.5–11 FFR is determined by inducing maximum hyperaemia with medications such as IV or intra-coronary adenosine. Deferral of PCI guided by wire-based FFR has also shown favourable long-term outcomes in studies such as FAME.8 When FFR is used to guide PCI, clinical outcomes are improved with fewer stents being deployed.8,12 Unfortunately, FFR remains underused in diagnostic and PCI procedures. A report from the CathPCI Registry of the National Cardiovascular Data Registry from 2011 (data collected from Jan 1 2010-Jun 30 2011) showed that only a third of patients had some form of stress testing prior to PCI.13 This could be related to factors such as time and cost.
Angiography-based ‘wire-free’ FFR is an emerging technique which determines the physiological significance of a coronary lesion without the need of a pressure wire or induction of hyperaemia. It is therefore useful in patients who cannot tolerate adenosine. It also eliminates potential complications associated with the introduction of wires into the coronary arteries.
In a systematic review of 536 patients in which 908 vessels were assessed, non-invasive FFR guided by CT had a reported accuracy of 82% for CT FFR values of <0.63 and >0.83, but only 46% for FFR of 0.70–0.80.14 In comparison, various wire-free FFR trials have shown high accuracy, sensitivity and specificity. Unfortunately, these trials were unable to directly evaluate the clinical outcomes using a wire-free FFR-based diagnostic strategy as decisions were still based on conventional FFR measurements.15–19 The accuracy of the wire-free FFR software algorithms in complex lesions is yet to be determined.
Wire-free FFR based on conventional coronary angiography emerged as an alternative to CT FFR as a means to measure FFR compaared to the gold standard method – FFR obtained from a wire introduced into the coronary artery, traversing the lesion to be interrogated.
There are numerous wire-free FFR systems available, many of which are now in clinical use. The quantitative flow ratio (QFR) system by Medis Medical Imaging has shown high sensitivity, specificity and accuracy. The 2017 FAVOR II China trial evaluated QFR in 308 patients from five centres and found a sensitivity, specificity and accuracy of 94.6%, 91.7%, and 92.7%, respectively.15 The concurrently run FAVOR II E-J trial evaluated 310 patients from 10 centres in Europe and 1 centre in Japan and reported a sensitivity, specificity and accuracy of 86.5%, 88.9% and 86.8%, respectively.16 Both trials also showed a superior sensitivity (88% versus 46%) and specificity (88% versus 77%) for detection of functionally significant lesions in comparison with 2D quantitative coronary angiogram using FFR as the reference. In-procedure wire-free FFR computation was feasible and possible within the time of standard FFR-based measurements but they did not assess clinical outcomes compared to wired strategies. A 2018 study of QFR, conducted by many of the same researchers, assessed 292 lesions in 191 patients. This trial demonstrated that QFR had a sensitivity, specificity, and diagnostic accuracy for predicting FFR of 77%, 86% and 83%, respectively.20 The explanation for the range of diagnostic characteristics in the studies is unclear.
The VIRTU Fast trial by Morris et al. in 2017 studied the virtual FFR (vFFR) system by Ansys CFX that used a pseudo transient mathematical model.17 This reduced the computational time from more than 36 hours to less than 4 minutes in comparison to the steady state mathematical model. The pseudo transient method computes transient results without the need for complex fully transient computational fluid dynamics analysis. The trial reported an accuracy, sensitivity and sensitivity of 100%. Further analysis also demonstrated that physiological lesion significance was determined less by coronary or lesion anatomy and more by coronary microvascular physiology. However, it required the use of an invasive pressure wire to compute microvascular resistance. It was also limited to 20 patients and required the use of rotational coronary angiography to produce a 3D reconstruction of the coronary artery.
The Fast FFR Trial used the FFRangio system by Cathworks to evaluate 301 patients using a lumped model system for processing data and reported a sensitivity, specificity and accuracy of 93.5%, 91.2% and 92.2%, respectively.18 A potential advantage of this system is the 3D reconstruction of the entire coronary tree with FFR values along each vessel. This may offer additional information to aid revascularisation strategies.
The Flash FFR system is a novel wire-free technique developed by Rainmed. The Flash FFR trial from 2019 showed high accuracy (96%), sensitivity (90%) and specificity (98%) compared to traditional wired-based FFR using the same cut-off value of 0.80 for significance.19 The coronary angiography FFR (CAFFR) system uses computational fluid dynamics (CFD) to solve the Stokes Navier equation and real time aortic pressure to determine the flow rate. 2D analysis of the angiogram is done to reconstruct a 3D image of the coronary artery to determine the FFR across a stenotic segment of the coronary artery. Originally, the computational fluid dynamics method was time-consuming and required more than 30 minutes of processing. In comparison, the mathematical analytical methods used for QFR and the lumped model for FFRangio took a significantly shorter time. The CAFFR system uses a specially designed CFD method to determine wire-free FFR with a processing time of 1 minute and an operational time of less than 5 minutes.19
The Flash FFR trial used this novel CFD method for the assessment of significance of coronary stenosis and to assess the diagnostic performance of the CAFFR system.19 However, the trial was not powered to assess the clinical implications of the CAFFR results in moderate coronary artery stenosis. The eventual treatment of these lesions was guided by the wire-based FFR. As a result, long-term clinical outcomes were not assessed in these patients.
The aim of our study was to assess performance of the CAFFR and the 12-month clinical outcomes of CAFFR-guided PCI deferral in intermediate coronary artery stenosis. To our knowledge, this was the first study of its kind in Southeast Asia.
Methods
This study was a prospective, single-centre study conducted at the Sarawak Heart Centre, Kuching, Malaysia from December 2019 to June 2020. In total, we assessed 69 patients (93 vessels) with angiographic stenosis of 30–90% and a vessel size of ≥2 mm in the stenotic segment by visual estimate. This was an all-comers study for patients indicated for coronary angiography and had no specific inclusion criteria. General exclusion criteria were if the patients were allergic to iodinated contrast, those ineligible for diagnostic intervention, severe coagulopathy or bleeding disorders or if the patient was participating in another trial. Angiographic exclusion criteria were if the interrogated stenosis was caused by a myocardial bridge, ostial lesions, significant vascular overlap, distortion of the interrogated vessel and poor angiographic image quality which prevented vessel detection. Due to either contrast streaking during the angiogram or significant vessel overlap resulting in inaccurate vessel mapping on the CAFFR console, suboptimal image quality excluded 12 patients and another eight patients were lost to follow-up. Of the remaining 57 patients whose data were analysed, 28 had CAFFR of ≤0.80 and underwent CAFFR-guided PCI. They were followed up at our clinic or at referral centres. The other 29 patients had CAFFR >0.80 and deferred PCI. Wire-based FFR was done as a comparison at the operator’s discretion, using the Abbott pressure wire X with the wire tip at least 3 cm distal to the lesion of interest. Hyperaemia was induced with intracoronary adenosine using at least 100 µg in the right coronary artery and 200 µg in the left coronary artery. A manual wire pullback was done to the guiding catheter tip to confirm the absence of pressure drift.
To compute CAFFR, two coronary angiograms of the artery of interest were performed at least 25 degrees apart with reasonable angiographic image quality and at a frame rate of 15 frames per second. Image acquisition was done with no X-ray magnification with the aim of capturing the entire vessel length in the same image field. Intravascular contrast injection through the guiding catheter into the coronary arteries was done at a rate of around 4 ml/s by stable hand injection. Mean aortic pressure (MAP) was measured using a pressure transducer (averaged over 5 cycles – from the third to the eighth cycle after angiography) connected to the guiding catheter (Flash Pressure, Rainmed). This was in keeping with the FLASH FFR trial from 2019.19 Contrast was injected via a 5 or 6 Fr catheter at the operator’s discretion. Intracoronary nitrates were given if there was a suspicion of coronary vasospasm but was not mandated as a prerequisite in all patients. Digital imaging and communications in medicine angiograms from two projections at different angles (≥25 degrees) and MAP from the pressure transducer were directly inputted into the CAFFR console via a local area network cable. A 3D reconstruction and mapping of the artery was performed along the vessel path from the ostium to the distal vessel based on the two angiographic projections. Vessel selection, mapping of the artery and determination of flow rate down the artery at rest was done by a single operator. Flow rate at a hyperaemic state, pressure drop and subsequent FFR across the stenosis was calculated in an automated manner by the CAFFR algorithm on the console.
All patients were prescribed antiplatelet therapy with either aspirin 75 mg daily or clopidogrel 75 mg daily and statin therapy with atorvastatin 20–40 mg alone or in combination with ezetimibe with an aim to achieve low density lipoprotein levels of <1.8 mmol/l. ß-blocker and angiotensin-converting enzyme (ACE) inhibitor were given in accordance with clinical practice guidelines. Patients who were treated with PCI were given a loading dose of clopidogrel 300 mg prior to intervention. After PCI, all patients received clopidogrel for a duration of 3–12 months in addition to aspirin 75 mg once a day along with optimal medical therapy. Aggressive secondary prevention of cardiovascular disease was done in all patients along with advice on smoking cessation. Smoking cessation clinics were arranged if requested. Patients with diabetes were closely monitored and referred to diabetes specialists if required.
All patients were followed up at 1, 6 and 12 months either by hospital clinic visits or telephone conversations. Information regarding angina, hospital admissions, repeat procedures and intervention were obtained. In patients that were reviewed in clinic, monitoring of cholesterol, diabetes and blood pressure was done to allow further drug titration.
The primary endpoint was a composite of all-cause mortality, MI and target vessel revascularisation at 12 months in patients who had CAFFR-guided PCI deferral.
Results
A total of 29 patients were included in the analysis for CAFFR-guided deferral of PCI. The mean age of our patients was 59.9 (±12.6) years, most of whom were men (82.8%; n=24). More than 60% of them had a smoking history; (62.1%; n=18) had hypertension and were either on medication or controlled it with lifestyle modification; 17 patients (60%) had dyslipidaemia and 12 patients (41%) had diabetes. The mean left ventricular ejection fraction was 53% (±11.4) and nine patients (31%) were known to have a history of coronary artery disease. An angiogram was done for a recent acute coronary syndrome in 22 patients (76%), 14 patients had a diagnosis of unstable angina or non–ST elevation MI and eight patients had a diagnosis of ST-elevation MI (STEMI) treated with IV thrombolytic therapy. In the STEMI cohort, seven (88%) had CAFFR assessment of non-culprit lesions. One patient had assessment of an infarct-related artery that was done more than 1 week after the index event. It is important to note that the time from the event to coronary angiogram was varied as most of these patients were from other referral centres.
In total, 31 lesions were assessed using CAFFR with the left anterior descending (LAD) artery assessed in 16 (52%) of the patients. The right coronary artery (RCA) was assessed in 12 (41.4%) patients while the left circumflex artery was assessed in four (13.8%) of the patients. The mean CAFFR was 0.87 (±0.04) in the LAD, 0.89 (±0.05) in the RCA and 0.88 (±0.04) in the left circumflex artery. There were no CAFFR-related complications. Wire-based FFR was done as a comparison to CAFFR at the operator’s discretion in roughly half (48%) of the patients.
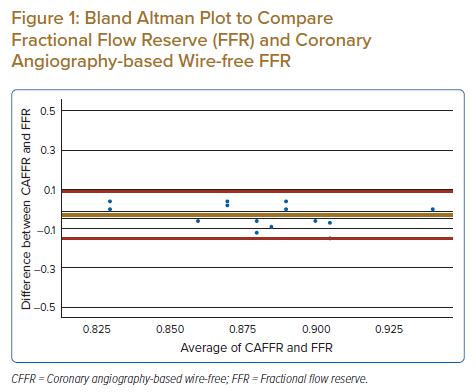
A Bland Altman plot (Figure 1) was done to analyse the performance of CAFFR and wire-based FFR. There was good agreement between the means and differences of CAFFR and wire-based FFR. There was no proportional bias as evidenced by a negative linear t-test with a p-value of 0.257.
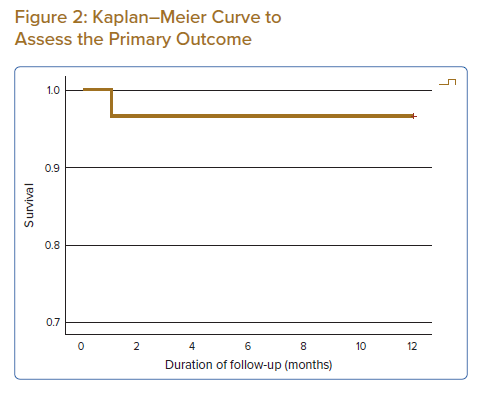
On follow-up at 12 months, none of the patients had died, but 28 (89.7%) remained in chronic coronary syndrome class 1. A Kaplan–Meier curve constructed for the primary outcome showed that only one patient met the primary outcome, representing 3.4% of the study population (Figure 2). This patient had a target vessel revascularisation for persistent symptoms of angina.
Discussion
The 12-month outcome showed that CAFFR-guided PCI deferral is safe and comparable to previously published trials on wire-based FFR. To our knowledge, this is the first study to assess CAFFR-guided PCI deferral in Southeast Asia and has the potential to aid further risk stratification of patients with intermediate coronary artery stenosis. The clinical implications of this may be significant especially in resource-limited environments.
In the patients that had wire-based FFR done for comparison, CAFFR showed good agreement with wire-based FFR. Our event rate of 3.4% at 12 months is similar to the PCI deferral group in contemporary wire-based trials such as the DEFINE FLAIR and iFR SWEDEHEART trial.21,22 It is worth noting that the patient who underwent target vessel revascularisation had an indication for stable symptoms and did not require hospitalisation.
This study has several limitations. It was a single-centre study with a small study population of 29 patients with eight patients lost to follow-up with their data excluded from analysis. This may be explained by the geographical distribution of our patient population in Sarawak. As some of our patients came from more rural settings, contacting them after the procedure was challenging. The follow-up period was only 12 months and outcomes could potentially change with longer follow-up. The CAFFR software has a learning curve and in order to eliminate inter-operator variability, we only used a single operator to run the software and determine CAFFR values for patients enrolled in the study. It is worth noting that the learning curve is not unlike other techniques we perform daily in our catheterisation laboratory.
Nonetheless, this is the first experience of a wire-free FFR using a combination of real-time angiography images and pressure measurements through its transducer system. Larger studies in different clinical scenarios with longer-term outcomes using this technology is warranted. Further studies comparing CAFFR-guided revascularisation and wire-based revascularisation would help in validating this new technology.
Conclusion
CAFFR showed good agreement with wire-based FFR and 12-month outcomes showed that CAFFR-guided PCI deferral was safe and comparable to wire-based FFR.
Clinical Perspective
- Coronary angiography-based wire-free fractional flow reserve (CAFFR)-guided percutaneous coronary intervention (PCI) deferral is safe.
- The 12-month outcomes in this cohort are comparable to previously published wire-based FFR trials.
- CAFFR-guided PCI deferral has the potential to further risk stratify patients with intermediate coronary artery stenosis. The clinical implications of this may be significant especially in resource-limited environments.
- Further studies comparing CAFFR-guided revascularisation and wire-based revascularisation would help in validating this new technology.