A 52-year-old man was being managed for symptomatic torrential tricuspid regurgitation (TR). He had Marfan’s syndrome with severe aortic regurgitation and extensive thoracoabdominal aortic aneurysms and dissections for which he had undergone multiple interventions. These included a Bentall procedure in 2002, thoracoabdominal aorta repair in 2011, aortic arch repair in 2012, thoracic endovascular aortic repair (TEVAR) in 2014 and endovascular aneurysm repair (EVAR) in 2019. In addition, he had AF, hypertension and minor coronary artery disease.
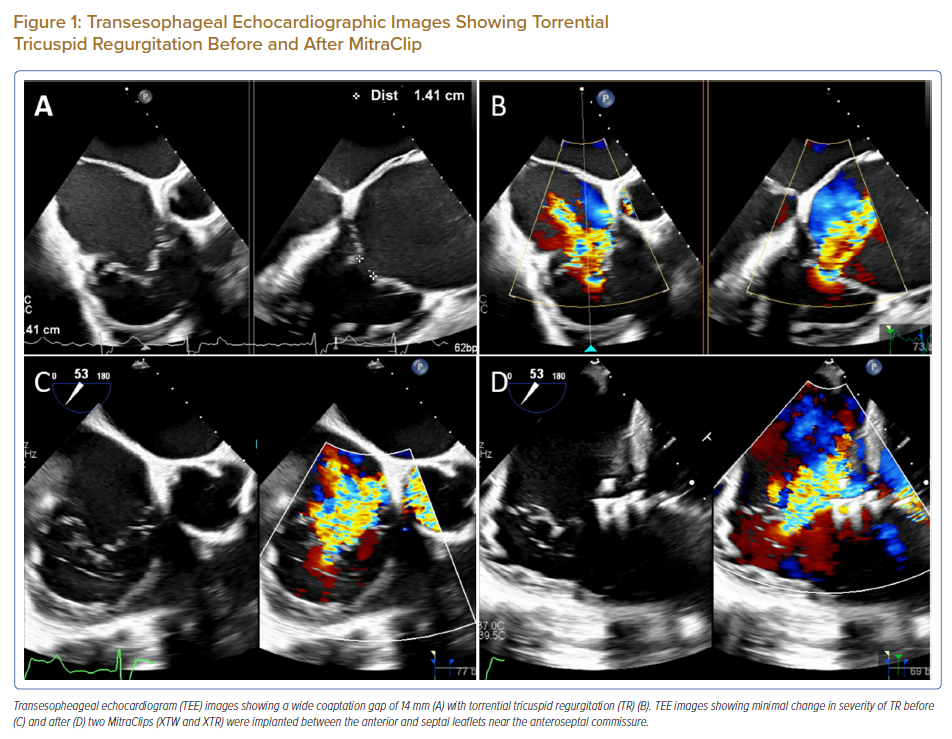
He started having worsening shortness of breath on exertion (New York Heart Association [NYHA] functional class III) and abdominal distension in 2020. Transthoracic and transesophageal echocardiogram (TEE) showed torrential TR (Figures 1A and B) with severe right ventricular (RV) dilatation (RV basal diameter: 68 mm) and impaired RV systolic function (tricuspid annular plane systolic excursion: 11 mm; tricuspid annulus S’ velocity: 9 cm/s). The tricuspid annulus diameter, right atrium (RA) area and inferior vena cava (IVC) maximal diameter were all dilated at 57 mm, 55 cm2 and 24 mm, respectively. Left ventricular size and function were normal and the aortic valve prosthesis was functioning well. Despite being on maximally tolerated medical therapy of furosemide (40 mg once daily) and spironolactone (25 mg twice daily) as well as therapeutic abdominal paracentesis for the ascites, he remained severely symptomatic and the ascites re-accumulated in a short period of time. He was deemed to be at high risk for open heart surgery having undergone two prior sternotomies, a thoracotomy and multiple other thoracoabdominal interventions. His Society of Thoracic Surgeons (STS) scores were 5.39% for mortality and 20.96% for mortality and morbidity, while his EuroSCORE II was 4.07%. His TRI-SCORE was 6/12 which gives a predicted in-hospital mortality of 22% after isolated tricuspid valve surgery.1 He then underwent transcatheter edge-to-edge repair (TEER) of the tricuspid valve with the MitraClip system (Abbott) in March 2021. Two MitraClips (XTW and XTR) were implanted between the anterior and septal leaflets near the anteroseptal commissure but they were unable to reduce the TR significantly due to the wide coaptation gap (Figures 1C and D; Supplementary Material Video 1). His symptoms persisted and he was subsequently considered for transcatheter bicaval valve implantation with the TricValve system (Products & Features) when it first became available in Singapore.
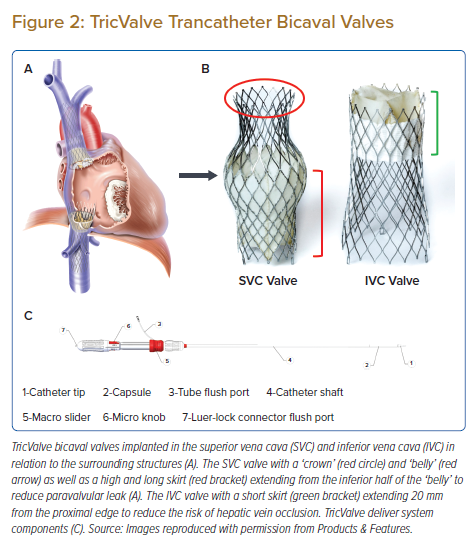
The TricValve system consists of two self-expanding bioprosthetic valves that are percutaneously implanted into the superior vena cava (SVC) and the IVC (Figure 2). It is used in the treatment of patients with haemodynamically significant TR by reducing reflux and congestion through the SVC and IVC to the other organs. Pre-procedural evaluation includes an echocardiogram, right heart catheterisation and CT scan. In addition to severe TR on echocardiography, there needs to be significant reflux into the SVC and IVC with a V wave ≥25 mmHg as demonstrated by right heart catheterisation when measured 2–4 cm above and below the inflow into the RA. Caval reflux is required for proper valve function after implantation. CT scan is required for valve sizing as well as to define the anatomy of the central venous system and its relationship with the surrounding structures.
The patient had markedly elevated SVC (V wave 37 mmHg; mean 24 mmHg), IVC (V wave 33 mmHg; mean 24 mmHg) and RA (V wave 36 mmHg; mean 23 mmHg) pressures consistent with the torrential severity of TR. The PA pressure was 43/15mmHg (mean: 28mmHg) and the RV end-diastolic pressure was 20 mmHg. Cardiac output was 4.86 l/min (2.55 l/min/m2) measured by Fick’s formula. CT measurements determined an SVC valve size of 29 mm and IVC valve size of 41 mm.
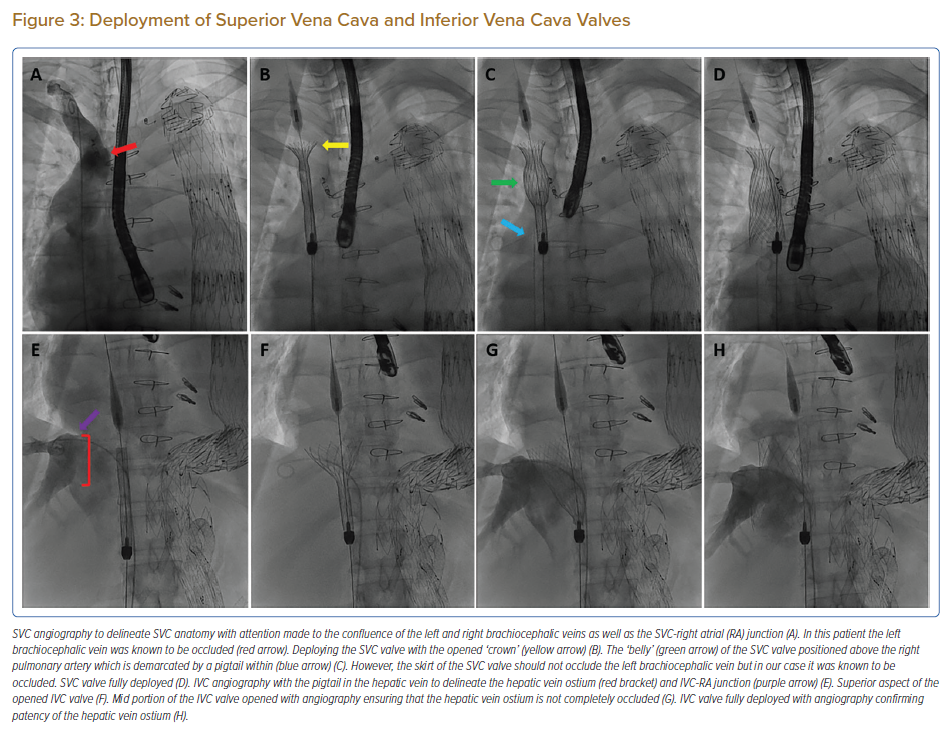
The procedure was performed in the cardiac catheterisation laboratory under general anaesthesia with fluoroscopic and TEE guidance. Intra-procedural blood pressure was monitored continuously with a right radial intra-arterial line. Bifemoral venous access was obtained. Two sheaths were placed in the left common femoral vein (CFV) – one for a pigtail catheter for contrast injection and another for a PA catheter to perform right heart pressure measurements and subsequently left in the right PA to serve as a marker for SVC valve implantation. The right CFV access was used for valve implantation. This was preclosed with two Perclose ProGlide (Abbott) vascular closure devices. From a 7 Fr access, it was sequentially dilated with 16 and 22 Fr dilators before introduction of the 27.5 Fr TricValve delivery system (Figure 2C). Before SVC valve implantation, SVC angiography was performed with a 5F pigtail located in the right brachiocephalic vein to delineate the SVC anatomy with attention made to the confluence of the left and right brachiocephalic veins and the SVC-RA junction (Figure 3A). The SVC valve should be implanted with the ‘belly’ of the valve above the right PA (demarcated with a pigtail catheter) and its skirt not obstructing the left brachiocephalic vein (Figure 3C). The patient’s left brachiocephalic vein was known to be occluded. The SVC valve was advanced and positioned using a 0.035 inch Amplatz Super Stiff guidewire. The micro knob was rotated clockwise (Figure 2C) very slowly to unsheath the SVC valve and to allow the nitinol frame to take the shape of the SVC (Figure 3B). There was a tendency for the valve to migrate superiorly and hence it was necessary to maintain some tension on the system. Just before complete deployment of the valve, the three connectors were released slowly by similarly rotating the micro knob clockwise. After releasing the SVC valve (Figure 3D), the delivery system was withdrawn into the IVC. The capsule was closed by either rotating the micro knob counter-clockwise or pushing the macro slider forward after unlocking (Figure 2C). Before IVC valve implantation, the catheter in the right PA was withdrawn and an IVC angiography was performed with the pigtail in the hepatic vein (see Figure 3E). The SVC valve delivery system was exchanged for the IVC valve delivery system. The IVC valve should be implanted as low as possible with respect to the IVC-RA junction to avoid paravalvular leak and contact with the inter-atrial septum. This positioning was guided by angiography but is frequently done using TEE. However, the skirt should not occlude the entire ostia of the hepatic veins and this was assessed with angiography during valve opening (Figures 3F and G). Similar to SVC valve implantation, the micro knob was rotated clockwise very slowly until all three connectors were released (Figure 3H). After IVC valve deployment, the capsule was closed before removing the delivery system and the groin was closed.
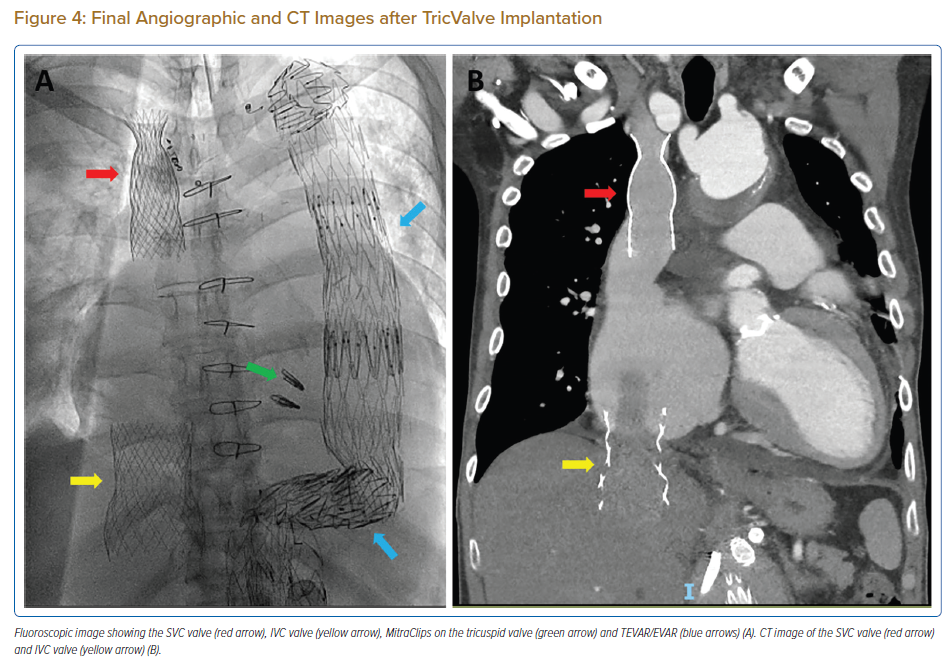
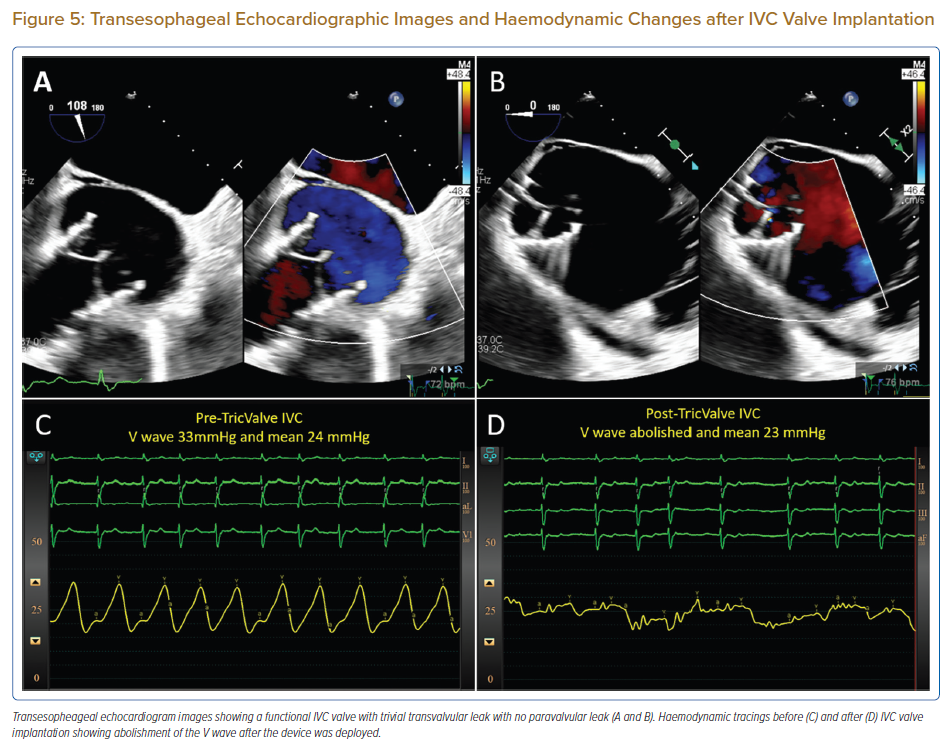
Post-implantation evaluation included angiography, echocardiography and haemodynamics (Figure 4). RA angiography did not reveal any paravalvular leaks (Supplementary Material Video 2). Visualisation of the SVC valve via TEE was challenging but the leaflets could be seen opening and closing well. The IVC valve was well visualised with trivial central leak and no paravalvular leak (Figures 5A and B; Supplementary Material Videos 3, 4 and 5). Post-procedure, the IVC V wave was abolished with a mean of 23 mmHg (Figures 5C and D) although the SVC V wave remained at 38 mmHg with a mean of 28 mmHg. The RA pressure increased with a V wave of 56 mmHg and a mean of 32 mmHg – which was expected due to the reduction of regurgitant blood flow into the venous system. PA pressure increased to 56/21 mmHg (mean = 34 mmHg) likely from the increase in preload, RV stroke volume and subsequent forward flow through the pulmonary circulation. Similarly explained, the cardiac output increased to 5.70 l/min (3.00 l/min/m2) measured by Fick’s formula.
The patient was extubated in the cardiac catheterisation laboratory and transferred to the high dependency unit for post-procedural monitoring. He experienced right shoulder pain which was referred pain from compression on the branches of the right phrenic nerve during IVC valve implantation as it courses through the caval foramen of the diaphragm. Pain relief was brought about by gabapentin, anarex and tramadol. He was stable at discharge and at 1 month, he had experienced significant improvement in symptoms to NYHA functional class I–II with a reduction in abdominal distention.
Discussion
Severe TR is associated with significant mortality and morbidity.2,3 In the past, treatment options were limited to diuretics for symptom relief and open-heart surgery where mortality rates were high.4,5 In recent years, transcatheter techniques have been developed to address this unmet need with TEER being the most commonly performed procedure.6 However, patients frequently present late at an advanced stage of the disease process where the RV is severely dilated resulting in severe tethering of the tricuspid leaflets and a coaptation gap that is too wide to allow effective TEER. In such cases, other transcatheter techniques are required.7,8 For the tricuspid valve, two categories of replacement technologies are available. In orthotopic valve replacement, the prosthetic valve is deployed at the level of the tricuspid annulus while in heterotopic valve replacement, it is deployed elsewhere such as the SVC or IVC in caval valve implantation (CAVI).
The concept of CAVI was first mentioned in 2006 by Davidson et al.9 The first-in-human CAVI was performed in 2010 with a self-expanding valve deployed in the IVC.10 The early experience of CAVI on 25 patients demonstrated the procedure’s feasibility (96% procedural success), safety (no intra-procedural mortality) as well as clinical (NYHA functional class) and haemodynamic benefits (reduction in mean IVC and RA pressures).11 In the past, the Sapien XT (Edwards Lifesciences) balloon-expandable valve (BEV) was used but there were challenges with sizing and anchoring in the dilated venous systems.12 In addition, the TRICAVAL randomised controlled trial comparing BEV and medical therapy was stopped early due to a high rate of valve dislocation requiring surgery.13 Dedicated devices such as the TricValve, Tricento (New Valve Technology, Muri, Switzerland) and Trillium (Innoventric) have since been developed.14
The TricValve system consists of two self-expanding bioprosthetic valves implanted into the SVC and IVC (Figure 2A). An important feature is that it does not interfere with the native tricuspid valve and it is therefore useful in patients who have previously undergone tricuspid valve interventions. The prosthetic valves have a frame made of nitinol, leaflets made from bovine pericardium and a skirt to prevent paravalvular leak (PVL). The SVC valve has a ‘belly’ in the middle and a ‘crown’ at the superior aspect to provide anchorage in the SVC. There is a high and long skirt that extends from the inferior half of the ‘belly’ to reduce PVL (Figure 2B). The IVC valve has a short skirt covering 20 mm of the proximal edge to reduce the risk of hepatic vein occlusion (Figure 2B).
There are some important considerations for CAVI. First, caval reflux needs to be demonstrated as pulsatile flow in the SVC and IVC is required for proper valve function. For this to be present, there needs to be significant TR and a certain preservation of RV systolic function. Second, lifelong anticoagulation is required to prevent device thrombosis after valve implantation in a slow-flow and low-pressure venous circulation. Third, there is huge inter-individual variability in the size of the caval veins and it can fluctuate further depending on volume status. It is therefore recommended to oversize the valve by performing the CT after omission of diuretics for 1–2 days before.
Certain issues may be encountered after CAVI. As mentioned in the case report, there can be referred pain to the right shoulder from compression of the branches of the phrenic nerve after IVC valve implantation. International experience reports this as self-limiting and responsive to analgesia.14 Device migration can occur especially during the peri-procedural period. Critical steps to avoid this include appropriate device sizing, meticulous positioning as well as slow and careful deployment of the device. In a post-mortem study of a patient who had CAVI 3 months earlier, the stent struts were found to be covered with fibrous tissue and firmly fixated to the caval vein indicating that the likelihood of late device migration is low.15 With the reduction of reflux into the caval veins, the RV experiences an acute increase in afterload, which may decompensate a failing RV, hence care must be taken in patients with baseline RV dysfunction but the precise degree of RV dysfunction that makes CAVI prohibitive has yet to be determined.
Conclusion
Patients with severe TR present at various stages of the disease process and more frequently at the advanced stages accompanied with multiple co-morbidities. The complex interplay between severe TR and different degrees of RV failure and dilatation brings about a huge spectrum of anatomical and physiological variability between patients that makes treatment selection challenging. Besides conventional surgery and diuretics, multiple transcatheter techniques have recently been developed and heterotopic valve replacement with CAVI has emerged as a valuable option in the armamentarium against TR. However, its eventual role in the treatment algorithm for TR among emerging therapies and its long-term clinical benefits still require further evaluation.
Clinical Perspective
- Severe tricuspid regurgitation (TR) is a challenging condition to treat with multiple transcatheter techniques being developed in an attempt to address the limitations of surgery and medical therapy.
- Caval valve implantation has emerged as a promising transcatheter option in the armamentarium against TR but its eventual role in the treatment algorithm and long-term clinical benefits require further evaluation.