Angiography is performed in the catheterisation laboratory to obtain images of coronary arteries to diagnose coronary artery diseases (CAD). Percutaneous coronary intervention (PCI) is an essential therapeutic invasive strategy in treating CAD. Both procedures require arterial access, including a femoral, brachial or radial approach. Compared with the femoral or brachial approach, transradial access (TRA) is associated with a lower likelihood of vascular complications than transfemoral access (TFA), but minor and major access site-related complications can occur following TRA.1
The use of TRA to perform diagnostic cardiac catheterisation procedures was introduced by Campeau in 1989, and 4 years later was adopted for therapeutic procedures in coronary angioplasty by Kiemeneij and Laarman in the Netherlands.2 In recent years, TRA for coronary intervention has become increasingly popular and has become dominant in several centres.3 TRA has emerged as the predominant route for performing coronary angiography and PCI worldwide, with growing use in the US and class I recommendations for its use over TFA in patients with acute coronary syndrome in the current European Society of Cardiology guidelines. In patients admitted with ST-elevation MI (STEMI) undergoing primary PCI, TRA reduces the risk of bleeding and vascular complication, increases patient satisfaction, and reduces in-hospital mortality.4
In Indonesia, a study that compared the benefit of TRA with TFA using data from nine hospital centres in 2017–2018 concluded that radial access was performed in 75% of all patients requiring percutaneous access.5 That study also showed that TRA has lower in-hospital mortality in patients with STEMI. In the RIVAL trial that randomised radial versus femoral access for coronary intervention, radial access did not reduce the primary outcome of death, MI, stroke or non-coronary artery bypass graft-related major bleeding compared with femoral access, but it showed reduced vascular access complications compared with femoral access, with similar PCI success rates, and was more commonly preferred by patients for subsequent procedures.6 Ando et al. also found reduced risk of acute kidney injury after catheterisation and intervention using TRA, favouring radial access in the PCI procedure.7,8
We report a case series in our institution on radial artery spasm (RAS) and perforation managed using the technique of balloon-assisted tracking and J-wire for kinking of the catheter, leading to a successful procedure.
Case Presentation
Case 1
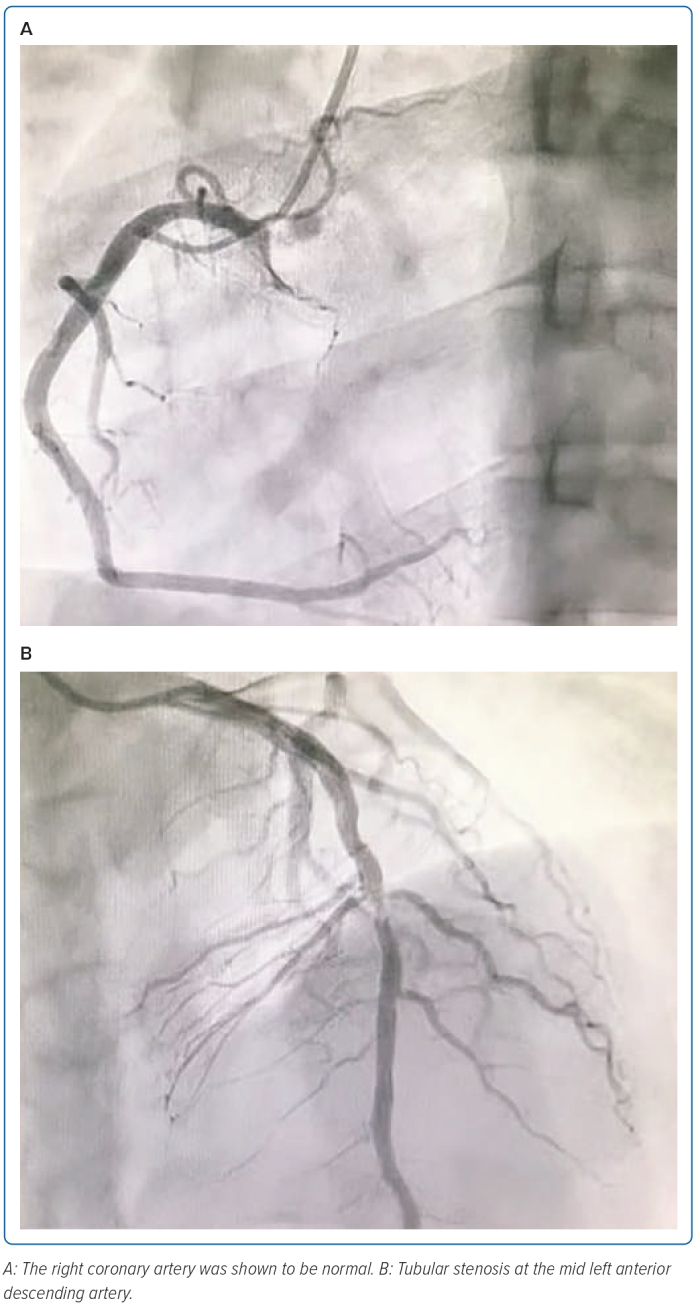
A 23-year-old man came to the emergency unit for 10 hours before admission due to chest pain. He experienced heavy-pressure chest pain for about >30 minutes when he was doing light activities. Nausea and diaphoresis were also present. His risk factors were active heavy smoking and hypertension. He was diagnosed with STEMI anterior onset 10-hour Killip 1 TIMI 3/14, and then we decided to undergo primary PCI. The patient was given lidocaine 2% and we proceeded to the radial puncture, and introduced a 6 Fr sheath. Standard spasmolytic (300 µg nitroglycerin) and heparin 8,500 IU were given intra-arterially. We successfully performed the cannulation of the right coronary artery (RCA) and left coronary artery with an Optitorque 5 Fr guide catheter (Terumo). The resulting coronary angiogram revealed 50% tubular stenosis in the mid left anterior descending artery with haziness (thrombus grade 1), whereas the left main coronary artery, the left circumflex artery and RCA were normal (Figure 1).
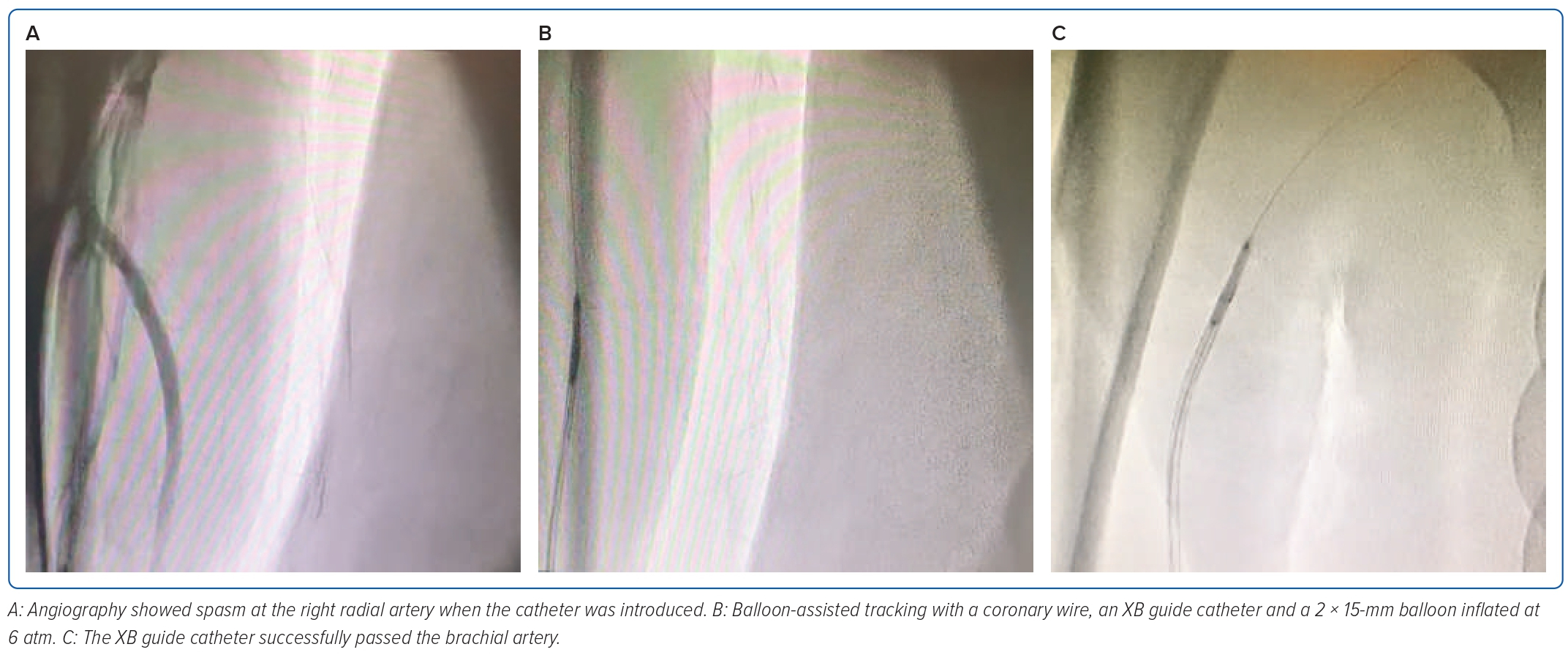
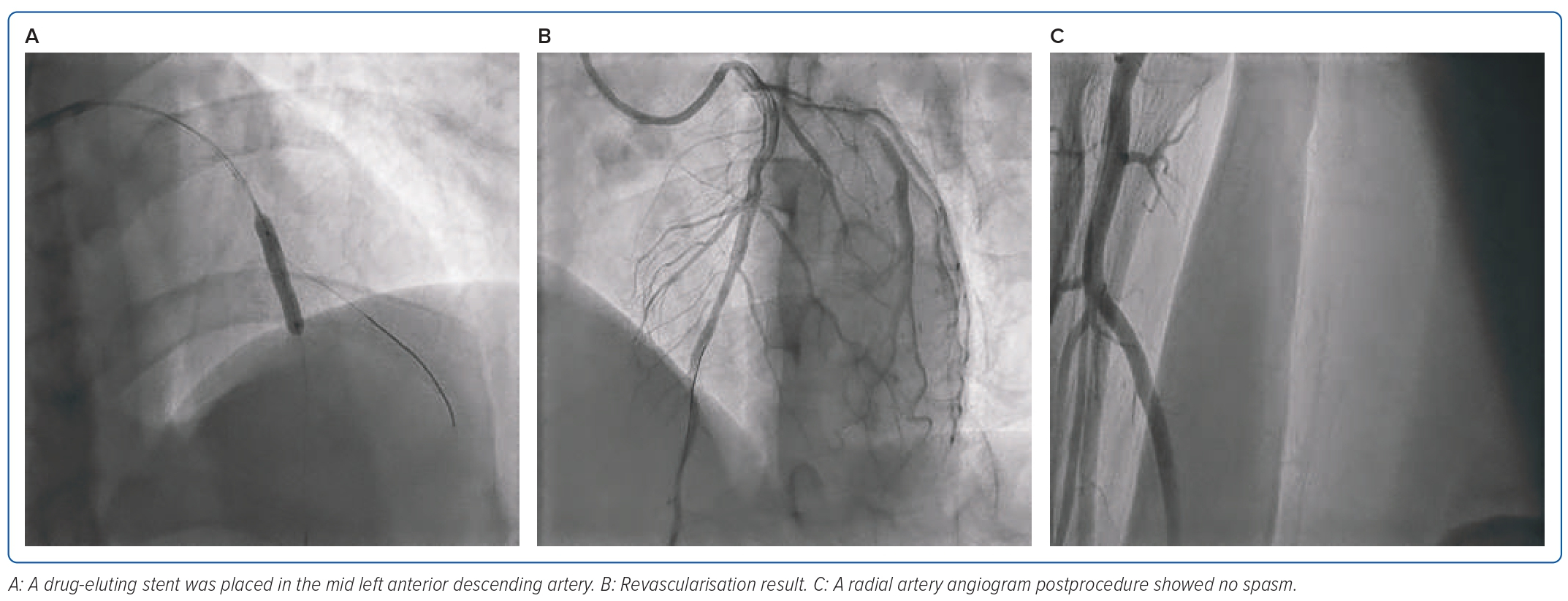
While the catheter advanced, there was resistance in the right radial artery. The angiography showed evidence of spasms. The patient also complained of discomfort in the forearm. An additional 200 µg of nitroglycerin was given without avail. We also tried to provide sedation with 25 µg of IV fentanyl without significantly improving the patient’s condition. We decided to perform balloon-assisted tracking (BAT) to advance the coronary wire using an XB 3.5/6 Fr guide catheter (Cordis). If the distal end of the catheter protruded with a 2 × 15 mm balloon, then the balloon was inflated with 6 atm. The XB guide catheter was successfully pushed up to the right brachial artery and exchanged with a J-wire Terumo. Finally, the stent was placed in the mid left anterior descending artery. The primary PCI procedure was successful, with minimal bleeding. A radial artery angiogram showed no spasm (Figures 2 and 3).
Case 2
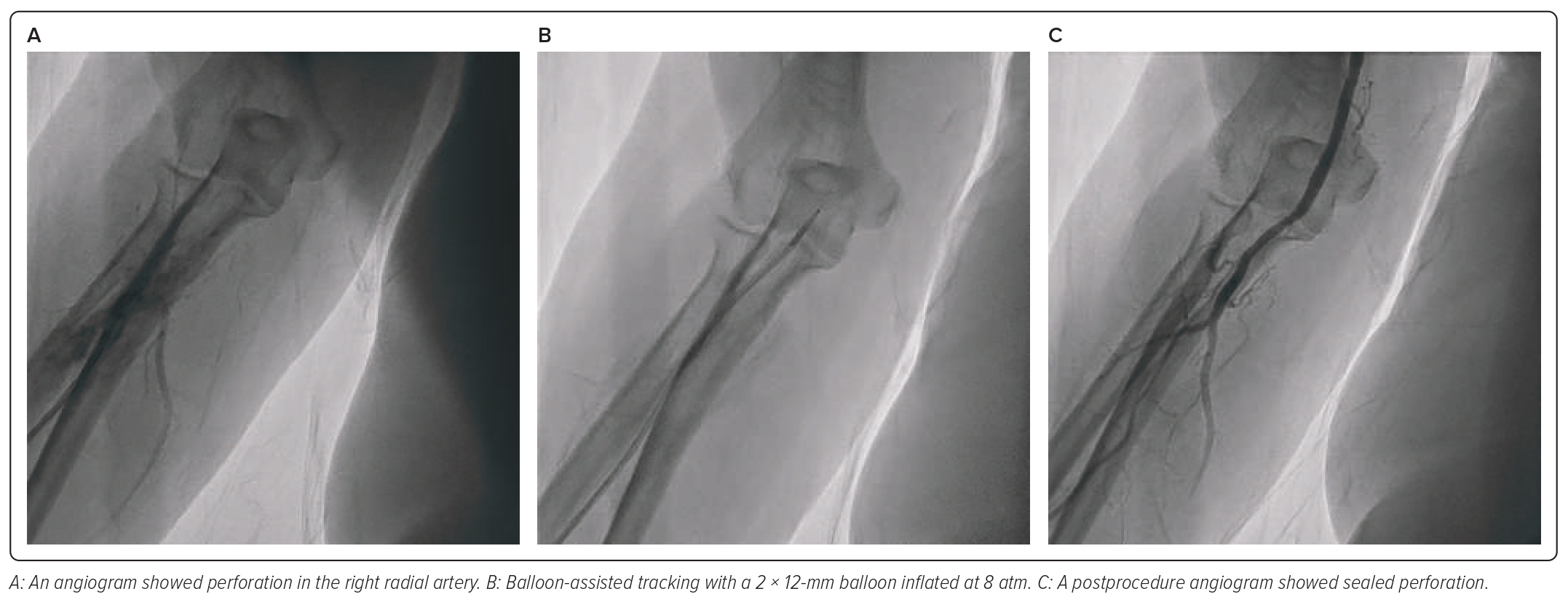
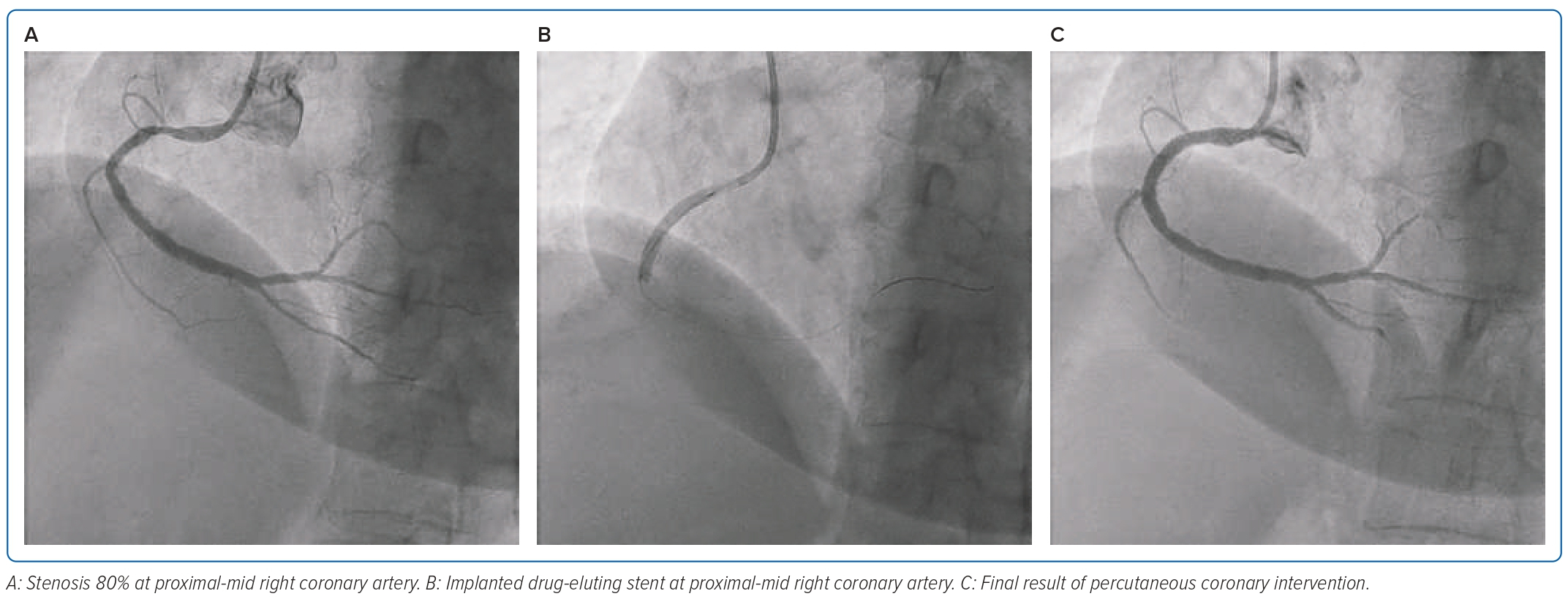
A 61-year-old woman came to an outpatient clinic with a history of recurrent chest pain induced by moderate activity. The complaints were relieved by taking sublingual isosorbide dinitrate. She denied any history of dyspnoea or palpitations. She had a history of a previous MI a few months earlier and had been admitted to another hospital. She also had coronary angiography 1 month earlier, with resulting stenosis 80–90% in the proximal RCA, while the left coronary artery was normal. She had risk factors, such as hypertension, menopause and dyslipidaemia. The patient underwent elective PCI using a 6 Fr radial sheath in the right radial artery. At the beginning of the procedure, there were no complications, until the guiding catheter was introduced, and there was resistance in the right radial artery. The patient also complained of pain in the forearm. The angiography result showed perforation in the right radial artery, then we decided to perform BAT. A coronary wire was placed with a compliant 2 × 12 mm balloon inserted into the guiding Judkins right 3.5/6 Fr catheter. It was advanced until the distal of the balloon protruded outside the tip of the catheter. The balloon was then inflated at 8 atm. BAT was successful and the guiding catheter could pass the radial artery. Diagnostic coronary angiography was performed using 6 Fr Judkins right 3.5. Angiography results showed 80% diffuse stenosis in the proximal-mid RCA. Then, with coronary wire, we implanted a drug-eluting stent 3.5 × 38.0 mm and inflated the balloon at 10 atm in the culprit area. The stent procedure in the RCA was successful. At the end of the procedure, we performed angiography of the right radial artery, which showed sealed perforation (Figures 4 and 5).
Case 3
A 61-year-old woman came to an outpatient clinic with a history of chest pain, lasting less than 5 minutes. This chest pain was triggered by moderate activity. There was no dyspnoea nor palpitation. She had risk factors, including hypertension, dyslipidaemia and type 2 diabetes, and was an ex-smoker. Treadmill tests in another hospital remained positive for an ischaemic response.
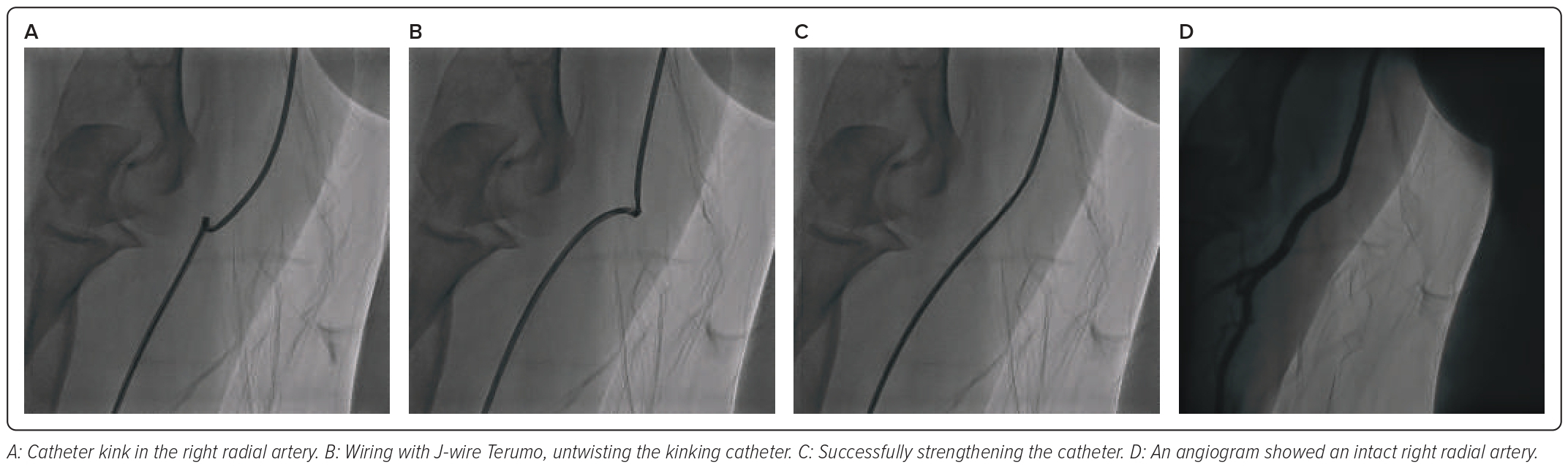
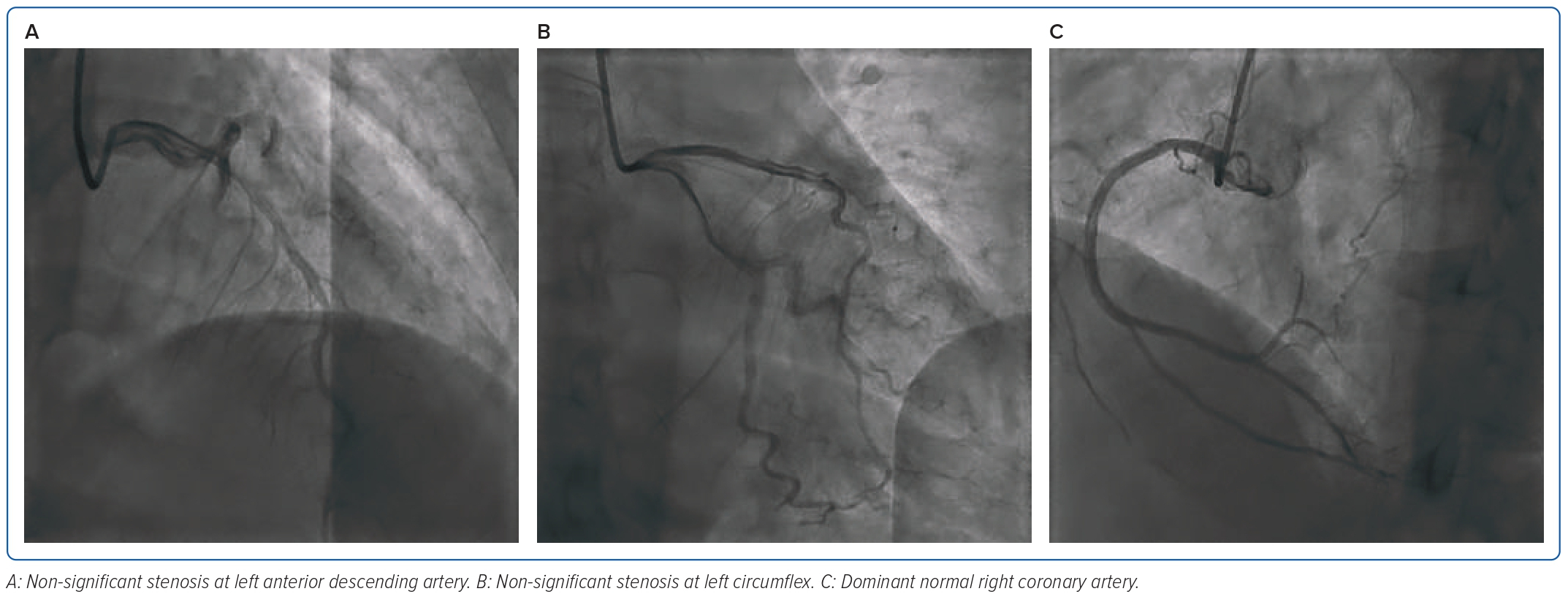
She was diagnosed with stable angina pectoris stage 2 chronic coronary syndrome, stage 2 hypertension and dyslipidaemia. The patient was scheduled for the elective coronary angiography procedure using a 5 Fr sheath. An Optitorque 5 Fr guide catheter was advanced. Catheter cannulation was deemed quite difficult, and damping pressure could be seen on the haemodynamic monitor. We tried an angiogram and found the problem was that the catheter was kinking in the region of the radial artery. We used a J-wire by untwisting the catheter in the opposite direction of the kinking catheter. This technique successfully passed the catheter and was successful in strengthening the catheter. We performed cannulation, and results were normal with dominant RCA, normal left main, non-significant stenosis in the left circumflex and non-significant stenosis in the left anterior descending artery. The conclusion was right dominant with non-significant CAD. The procedure went successfully, with minimal bleeding (Figures 6 and 7).
Discussion
The most critical portion of the catheterisation procedure is attaining vascular access. Access can be obtained in the femoral, brachial and radial arteries. Some of the critical points of vascular access may not seem important, but those steps are crucial to the safety and success of the procedure. TRA is still the preferred approach for coronary angiography and PCI, because, when compared with TFA, this approach can reduce the risk of bleeding and vascular complications, increase patient satisfaction, and reduced mortality in patients presenting with STEMI compared with the TFA. Although femoral access is considered to be quicker and easier, this vascular approach has more complications. Radial access is considered to have a steeper learning curve, but has minimal complications.
A study by Alkatiri et al., based on a study from nine hospital centres in Indonesia, stated that femoral access has a higher incidence of developing cerebrovascular accidents, cardiogenic shock and life-threatening arrhythmia in patients undergoing emergent PCI during their hospital stay. Otherwise, radial access showed reduced in-hospital mortality in STEMI patients. Besides that, there are also disadvantages of the radial approach, including increased radiation exposure to the operator during the learning curve, limitation of guide catheter size in some patients and radial artery occlusion.1,4,5 One of the complications of TRA is radial artery occlusion. Based on the DISCO RADIAL trial, the use of distal radial access showed fewer incidents of radial artery occlusion than conventional TRA (0.91 versus 0.31%; p=0.29).9
Based on meta-analysis on a randomised controlled trial by Ferrante et al., major bleeding occurred in just over 1% of cases. There are also several complications that may occur in TRA, such as radial spasms, perforation, pseudoaneurysms, arteriovenous fistulas and haematomas. The highest major vascular complication rate (1.4%, 49/3,507) was reported in the RIVAL trial, where most were due to large haematomas (n=42).6,10
Radial Artery Spasm
RAS is the temporary, sudden narrowing of the radial artery. The incidence of RAS is 4–5% in large, randomised control trials (RADIAL and RIVAL). The radial artery reflects a higher sensitivity for spasms than other bodily vessels, then expresses primarily α1-adrenergic receptors with fewer β2-adrenergic receptors; the radial artery is extremely sensitive to catecholamines and prone to spasms if directly or repeatedly stimulated by a guidewire.11,12
Younger age, female sex, diabetes, lower BMI, multiple puncture attempts and use of larger introducer sheaths (7 Fr) are independent predictors of RAS. Furthermore, small radial artery diameter, large sheath-to-artery size ratio, excessive manipulation of intra-arterial wires and multiple catheter exchanges can trigger RAS. Most RASs are mild, and multiple techniques can be used to prevent and treat them.13
To reduce the possibility of multiple punctures, a systematic review showed the usefulness of ultrasound-guided radial artery access in increasing first-pass success and reduced failure rates.14,15 Adequate pain control and sedation are the keys to preventing RASs. Deftereos et al. reported that patients with adequate sedation and analgesia had a lower incidence of RAS compared with patients without adequate sedation (8.3 versus 2.6%; p<0.001) and less frequent access site cross-over (15 versus 9.9%; p=0.001).12 RAS often manifests with forearm discomfort following resistance to the wire, sheath or catheter advancement, and may worsen with further manipulation. The most frequent adverse consequence of RAS is the need to cross over to another access site.16
There are several strategies to prevent RAS both pharmacologically and non-pharmacologically. Pharmacologically, prevention includes administering nitroglycerin (sublingual/subcutaneous/topical) before the arterial puncture. However, the use of nitroglycerin has some relative contraindications, such as severe aortic stenosis, haemodynamic instability or hypertrophic cardiomyopathy. A recent meta-analysis concluded that 5 mg of verapamil, with or without nitroglycerin, was the most effective and frequently used spasmolytic agent. Non-pharmacologically prevention, including forearm heating, such as manual heating using the operator’s palm (Balbay manoeuvre) for 3 minutes after administration of 1% lidocaine for local anaesthesia subcutaneously and warm compresses (using surgical towels sank into warm water, or warm blankets) for 3 minutes can be used. Flow-mediated dilatation, where a sphygmomanometer cuff placed around the mid-upper arm and inflated 30–50 mmHg above systolic blood pressure can be used.12
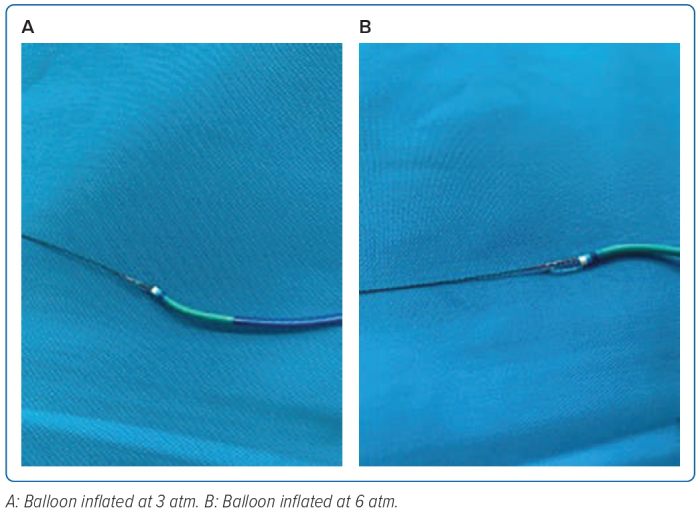
Another method is cannulation using BAT, as done by Veruden et al. In their report, the use of BAT resulted in uncomplicated engagement into the ascending aorta, due to the conical shape of the protruding balloon with its coated surface, along with low-pressure inflation (3 bar), which then can be increased to medium-pressure inflation (6 bar) to overcome spasms.17
In the first case, we found RAS. As stated before, a young age is one of the risk factors for RAS. For this complication, 300 µg of nitroglycerin intra-arterial was administered, without success. We proceeded to perform BAT with coronary wire and a balloon 2.0 × 15 mm inflated with 6 atm pressure. XB guide catheter successfully pushed up to the right brachialis artery and exchanged with a J-wire, and then the cannulation was successful. After the procedure, angiography in the brachialis artery region showed no spasm or perforation. This case showed the effect of BAT by using the tip of the balloon catheter by inflating the balloon at 6 atm, increasing its pushability effect, so it was easier to traverse through severe RASs (Figure 8).
Radial Artery Perforation
Radial artery perforations are more likely to occur in elderly, people of short stature, women and those with small or tortuous vessels. They can be caused by radial sheaths, guidewires, diagnostic catheters or guiding catheters, and are described in <0.5% of cases. Radial artery perforation is often diagnosed immediately. Forearm pain or vasovagal reactions following complicated wiring or catheter advancement should prompt immediate angiography. Radial artery perforation can cause serious bleeding, which, if severe, or not managed appropriately, can lead to compartment syndrome and require surgical intervention.
There are several options for the standard management of radial artery perforation, including applying pressure either manually or with the use of a sphygmomanometer, resulting in the stasis of blood. Most perforations can also be managed conservatively by sealing the perforation site by advancing a long sheath or a guide catheter to seal the perforation. Some operators report prolonged balloon inflation at the site of the perforation and treatment with polytetrafluoroethylene-covered coronary stents are used to seal the perforation. The wire should pass the perforated segment of the radial artery as the key to the success of all these techniques. Suppose the wire is not across or unable to cross, in that case, immediate haemostasis by inflating a sphygmomanometer proximal to the perforation, compression with an elastic bandage or manual compression at the level of systolic blood pressure, and protamine should be pursued to prevent significant blood loss, haematoma formation, compartment syndrome and the need for surgical intervention, which is rare.11,18
Another option for managing this complication is BAT, which allows the procedure to continue via the affected arteries and successfully seal the perforation.19,20
In the second case, the patient came to the outpatient clinic due to a history of recurrent chest pain induced by moderate activity. Risk factors found in this patient were female sex, short stature and hypertension. Coronary angiography found one-vessel CAD and stenosis in the RCA. This patient decided to undergo elective PCI. During the procedure, there was a resistance in the right radial artery, and the patient also complained of pain in the forearm. Then, we performed angiography in the arm and found a perforation in the right radial artery. There was an active extravasation of contrast. After that, we decided to perform BAT with a balloon emerging 2 × 12 mm, and the balloon was inflated until 8 atm. BAT was successful by using the tip of the balloon. However, after full evaluation of the patient, we believe it is more suitable to perform inflation at low pressure of approximately 3 bar to use its flexibility effect, helping to eliminate the ‘razor’ effect to overcome the perforation segment.
Catheter Kinking
Another intraprocedural challenge that can occur, leading to complications, is catheter kinking. Catheter kinking is more common in the femoral procedure, but this can also happen in the radial procedure. Kinking can occur at the level of the radial, brachial, subclavian or axillary artery. Loss of pressure waveform, unresponsive catheter, limited wire movement, limitation of torqueability or enhanced resistance to injection are all signs of catheter kinking. Once the catheter twist is confirmed, it must be relieved by torquing it gently in the opposite direction under fluoroscopic guidance. Should this fail, introducing a guidewire or percutaneous transluminal coronary angioplasty wire is conducted across the twisted segment. After untwisting, the catheter must be removed and replaced with another catheter. If this simple measure fails, a more advanced procedure, such as external compression of the upper arm with a sphygmomanometer at supra-systemic pressure or a mother-and-child technique, can be attempted. If all such measures fail, using a gooseneck snare via femoral access to grasp the catheter tip may help as an advanced technique. If all the techniques fail, the patient should be subjected to surgical removal of the twisted catheter.21,22
In the third case, the patient was diagnosed with stable angina pectoris, stage 2 chronic coronary syndrome, stage 2 hypertension and dyslipidaemia. She underwent coronary angiography, but there was difficult cannulation and damping pressure during the procedure. As described before, loss of pressure waveform, the catheter becoming unresponsive, limited wire movement to the kink area, limitation of torque and enhanced resistance to injection were found. Then, an angiogram was performed to find the problems. The angiogram picture showed kinking in the radial region; by using a J-wire, we untwisted the catheter kink.
Conclusion
We presented three cases of radial artery complications during coronary angiography and elective PCI procedures, which were managed by different approaches. Early recognition, contemporary prevention and management techniques may help to reduce the occurrence and severity of complications related to TRA. 
Clinical Perspective
- Transradial access is currently the dominant route of access in coronary intervention. Although relatively safe to use compared with femoral access, there are some complications that can occur, such as radial artery spasm, perforation and catheter kinking.
- There are several ways to overcome radial artery spasm, divided into pharmacological and non-pharmacological.
- In a case presented here, the use of balloon-assisted tracking was proven to be able to overcome radial artery spasm and radial artery perforation as a complication in transradial access.
- There are several steps in the management of catheter kinking, such as untwisting and inserting wire, using a snare catheter tip, indeflator gentle inflation and surgical removal.