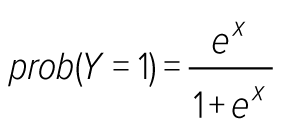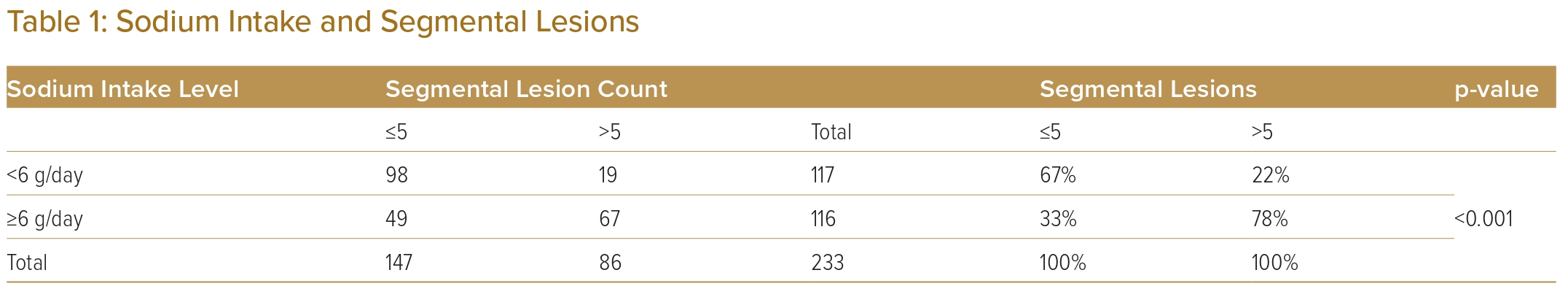With ongoing demographic and epidemiological changes, the burden of chronic non-communicable diseases (NCDs) is rising globally, including in Sri Lanka.1 Cardiac diseases, including ischaemic heart disease (IHD), are among the main conditions attributed to the chronic NCD burden and are one of the leading causes of hospital deaths in Sri Lanka.2,3 The spectrum of presentations of IHD includes stable and unstable angina, MI and sudden cardiac death, which are also associated with plaque formation.4,5
Most patients with IHD present with other comorbidities, such as hyperlipidaemia and diabetes, increasing the morbidity profile. Atheroma is the reversible, asymmetric deposition of degenerative material in the innermost layer of the arterial wall. The deposited material consists of fibrous connective tissue, lipid and calcium debris and macrophages. These substances cause swelling in the arterial wall that protrudes into the lumen of the artery, ultimately narrowing it and restricting the blood flow.6
As regards atheroma formation in arterial walls, the death of macrophages appears to be an essential contributor to necrotic core formation and plaque destabilisation.7 Macrophages are large, specialised cells formed in the immune system in response to infections or accumulation of damaged or dead cells and are an essential part of the defence mechanism against infection. Macrophage death in atherosclerosis includes passive apoptosis or accidental necrosis. Efferocytosis can also cause secondary necrosis, whereby the phagocytic process insufficiently clears apoptotic cells. These processes may be either beneficial or harmful in atherosclerosis.8
Statins have significantly reduced atherosclerosis-associated morbidity and mortality and they have beneficial actions beyond lowering plasma cholesterol levels. The effect of statins on interferon-γ-induced macrophage gene expression in human macrophages has also been analysed.9 Cell membranes are primarily composed of fatty-acid-based lipids and proteins, with the membrane lipids primarily consisting of phospholipids and sterols (generally cholesterol).10 Statins lower the risk of premature death, heart attack and stroke, regardless of serum cholesterol levels and even among individuals with relatively normal cholesterol (the so-called pleiotropic effect).11
Sodium concentrations in body compartments are closely regulated. For example, sodium chloride concentrations are higher in the interstitial fluid than in the plasma fluid by 2 mmol/L (Na) and 12 mmol/L (Cl).12 Sodium can cause changes in blood vessels, primarily by acting on the endothelium, which lines the lumen and originates from the mesoderm.13,14 Sodium enters the apical surface of the endothelium along a concentration gradient through sodium-glucose and sodium-amino acid symporters. The sodium-potassium pump in the basolateral region is an active process that consumes adenosine triphosphate and can increase interstitial sodium concentrations.15 WHO recommends consumption of less than 5 g of salt per day for adults (aged 18+ years).16,17 At higher concentrations, the osmotic action of salt has a coagulative (denaturalising) effect on proteins; however, whether the effect is concentration dependent is unclear.17 When a protein is denaturised, its structural changes as a result of salt cause the protein to lose its biological activity. When denaturation occurs, the secondary, tertiary, or quaternary structures are significantly affected.18 Following organ injury or insult, the immune system is essential for the onset, progression and resolution of inflammation. The main controllers of tissue fibrosis, regeneration and repair are tissue-resident macrophages and inflammatory monocytes.19 Continual exposure to this can lead to an atheromatous plaque formation as macrophage is a main component.8
The walls of arteries close to the heart are the thickest and can withstand the high-pressure flow of blood ejected during systole. However, these arteries contain a high percentage of elastic fibres in all three tunica layers, enabling expansion and recoil after each pumping action of the heart. Thus, they are known as elastic arteries. If they were rigid, their resistance to blood flow could increase blood pressure.20 Although these arteries are distensible and will stretch at high pressure, the flow within them will increase linearly as in a rigid tube, the adventitia limits the degree of distensibility. There is more proof that the adventitia plays a role in vascular contraction and relaxation from other studies that used rat aorta, carotid and iliac arteries and performed surgical layer separation21
The tertiary care unit of Sri Jayewardenepura General Hospital (SJGH) has performed cardiac surgeries since 1992. Because SJGH receives referrals from across Sri Lanka, it has become an ideal location to study IHD.
The need for more attention on NCDs, including cardiovascular diseases, is widely recognised in Sri Lanka because of rising disease trends. From the mid-1970s to 2006, the rate of hypertensive disorders and diabetes increased by more than threefold and IHD by more than fivefold.22 Heart disease, diabetes and high blood pressure are the three diseases with the expected exponential increase in hospitalisation between 2005 and 2010 and beyond.23 In the upcoming years, diabetes and coronary artery disease are predicted to be the main causes of morbidity and mortality. Just over half of those with heart disease, diabetes and hypertension (55.6%) had received drug therapy and counselling to prevent heart attack and stroke.23 Therefore, factors influencing cardiovascular disease in Sri Lanka must be identified.
The technical report on sodium intake and cardiovascular disease in low- and middle-income countries states that high sodium intake (>5 g/day) is associated with an overall increased risk of cardiovascular disease.24 Furthermore, in 181 of 187 countries surveyed (99.2% of the global adult population), the mean sodium intake exceeded the WHO-recommended maximum limit of 2,000 mg/day.16 Therefore, sodium intake levels must be studied, including their possible association with IHD occurrence in the Sri Lankan context.
This study aimed to estimate sodium intake levels and describe the association between salt consumption and atheroma formation among patients undergoing coronary artery bypass grafting (CABG) at SJGH in Sri Lanka.
Method
This descriptive cross-sectional study was conducted at the cardiothoracic unit at SJGH. Data were collected from October 2019 to August 2020. All 300 patients who had been admitted to SJGH for CABG during the data collection period comprised the study population, including all patients with medically optimised comorbidities. The minimum sample size at the data collection stage was estimated based on the average sodium intake of people using the following formula.25 According to a study in Sri Lanka, the average daily salt consumption is 8.3g (SD 4.3; 95% CI [7.9–8.8]).26
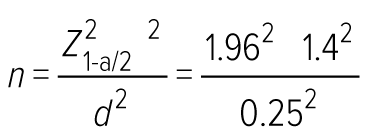
where n is the sample size
Z1−a/2 = 1.96 (at 5% significant level)
σ is the standard deviation of sodium intake of a person (estimate) = 1.4
d = absolute error = 0.25 g/day
Therefore, the calculated minimum sample size was 120. A lower absolute error was used to obtain the highest sample size.
Data collection was conducted with an interviewer-administered questionnaire, which included four sections: participant characteristics, past medical history, details on food intake including salt consumption and affected coronary arterial segment. Picture aids were used in the food intake section. Salt consumption was calculated in relation to salt used at home, processed food with added salt and food eaten in restaurants. Home-based salt consumption was calculated with the meal frequency in the previous month and the dispensed amount of salt in a meal. The salt content of processed foods and restaurant food was calculated using web-based applications and food tables.27,28 The distribution of coronary segments with atheroma based on the participant’s angiogram report was provided by the SYNTAX score website (https://syntaxscore.org; Supplementary Material Table 1).29 The number of segments affected was counted, without considering the number of plaques in a segment.
Three investigators collected the data. The research design was based on two levels of IHD (high: >5 segments involved; low: ≤5 segments involved) and the number of segmental lesions found in patients with IHD. Univariate and multivariate analyses were used to analyse the binary outcome of the occurrence of two levels of segmental lesions (dichotomised), with the effects of possible risk factors as independent variables. The distribution of segmental lesions in the sample ranged from 0 to 9, with the average number of segmental lesions being five.
Based on the central tendency, segmental lesions were classified as low for ≤5 segmental lesions and high level for >5 segmental lesions. To identify the potential risk factors associated with IHD occurrence, selected demographic and lifestyle-related risk factors were examined with both univariate and multivariate analysis. In the univariate analysis, associations of categorical variables between patients with two levels were determined using the chi-squared (χ2) test or Fisher’s exact test, as appropriate, and a two-sample t-test was applied to compare the means of BMI and waist circumference in the two levels of IHD. In the multivariate analysis, a logistic regression analysis was used to identify variables significantly associated with IHD and adjust confounding variables. Therefore, the logistic regression model was as follows:
where Y is the binary dependent variable Y = 1 for ≤5 segmental lesions or Y = 0 for >5 segmental lesions).
Z = β0 + β1x1 + β2x2 + ∙∙∙ + βnxn
where each xi is a predictor and each βi is the regression coefficient. ORs were calculated as a measure of relative risk of each factor. The CI estimates for the slope and intercept were determined on their relative Wald test. Statistical analyses were performed using R software version 4.1.1, which was developed by the R Core Team. The association between the number of segmental lesions and serum sodium levels of patients were tested with the Pearson correlation coefficient.
Results
Recent studies have estimated that Sri Lankans are consuming higher than the recommended 5g of salt per day.26,30 Table 1 shows the relationship between the two levels of IHD based on the number of segmental lesions, sodium intake levels with percentages and p-values of the χ2 test. Sodium intake shows a significant association with the number of segmental lesions (p<0.001; Table 1). Supplementary Material Table 2 shows the number of observations in the two levels of IHD and other demographic and lifestyle-related variables with the percentage and p values of the χ2 and Fisher’s exact test. A few recent cohort studies have reported a J-shaped relationship, that is, both lower and higher sodium intake levels were associated with an increased risk of cardiovascular disease events and mortality.17 In addition, diabetes showed a significant association with the IHD disease levels (p<0.10; Supplementary MaterialTable 2).
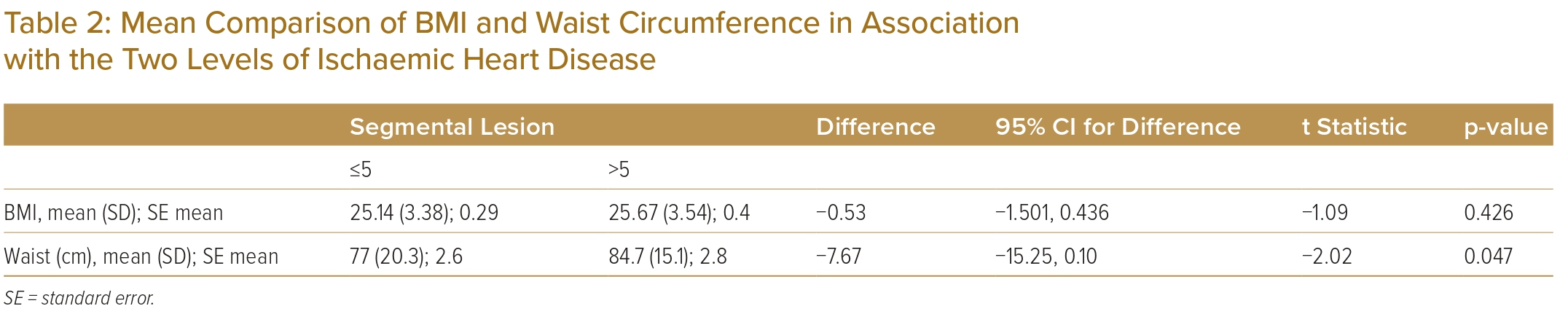
As shown in Table 2, the average waist circumference is significantly higher in patients with >5 segmental lesions (p<0.05), although BMI does not show a statistically significant difference between the two levels of IHD.
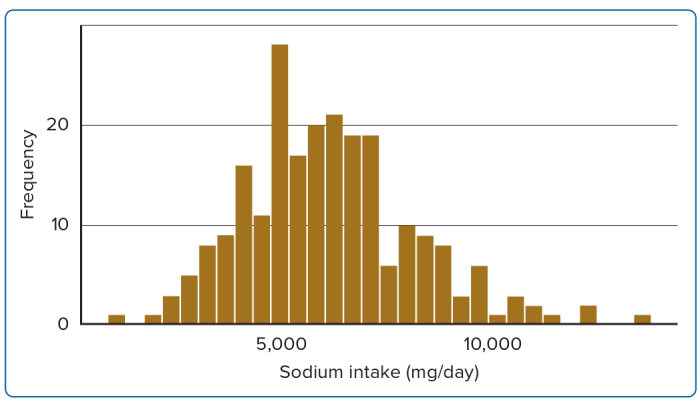
Considering the above analysis, the influence of dietary sodium intake can be more widely recognised as a significant risk factor for IHD. Figure 1 shows the average distribution of dietary intake of sodium per day. Accordingly, the average intake of sodium per person is 6,176 mg/day, despite the WHO-recommended level of 2,000 mg/day, which exceeded the WHO-recommended upper limit of sodium intake.
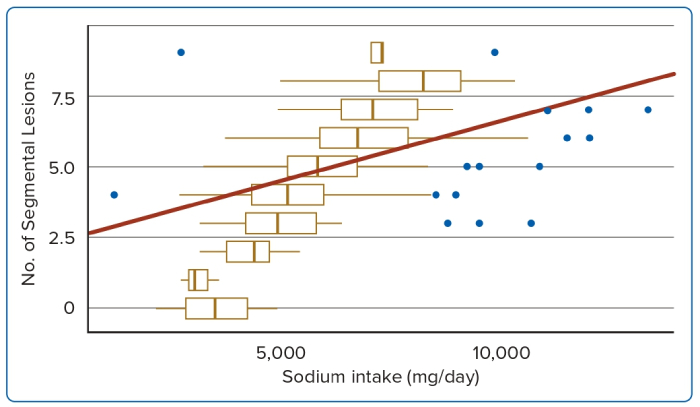
Further analysis of segmental lesions versus sodium intake provides significant evidence and the fitted regression line (p<0.05) is as follows (add R2 = 25.0%): number of segmental lesions = 2.443321 + 0.00042 sodium intake (mg/day). Figure 2 illustrates the relationship between sodium intake and the number of segmental lesions and the distribution of segmental lesions over daily sodium intake with boxplot diagrams.
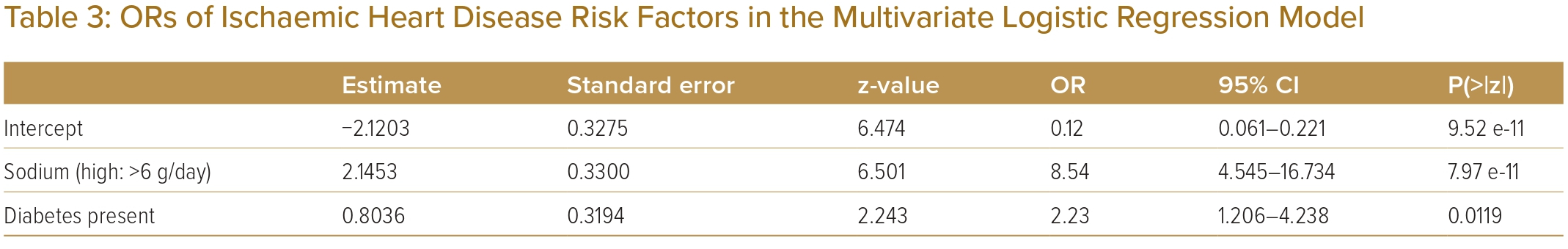
According to the findings, the most important elements are sodium intake and presence of diabetes affecting the frequency of segmental lesions. Furthermore, the multivariate logistic regression model demonstrated the influence of high sodium intake (OR 8.54; 95% CI [4.545–16.734]) on diabetes (OR 2.23; 95% CI [1.206–4.238]) while contributing to the main risk factors for IHD.
As per the Pearson correlation between the number of segmental lesions and serum sodium, the correlation coefficient was −0.074 (p>0.05) and no evidence for a strong relationship was noted. This may be because all participants were medically optimised; thus, the serum sodium concentration may not reflect the effect of serum sodium on IHD.
Discussion
This study looked at the factors associated with atheroma formation with special emphasis on salt intake and helped establish the relationship between sodium intake (when calculated using salt intake) and atheroma formation (p<0.01). Even when adjusted for confounders, the significant association between salt intake and atheroma formation is evident. In this study, dietary history was obtained with multiple verifications and sodium intake was calculated based on dietary history extracted from patients by the investigator.
The OR of sodium intake was 8.54 and patients with >5 segmental lesions are 8.54 times more likely to be consuming high levels of sodium (≥6g/day; Table 3). BMI was found to have no significant effect on the low number of segments (in the mean comparison with two-sample t-test analysis). In a study conducted in Northern Iran, BMI and waist circumference were not independently related to the severity of atherosclerosis in adults.31
However, according to the binary logistic regression analysis, cholesterol levels did not show a significant contribution (p>0.05). Since table salt consumption contributes to sodium and chloride intake, cholesterol and chloride appear to contribute to the incidence of acute heart failure, demonstrating a significant positive correlation in serum chloride and total cholesterol concentrations as demonstrated by Radulović et al.32 Probably, chloride initiates hyperlipidaemia, which contributes to atheroma formation. Sodium is an essential element in human biology and it is used in food worldwide. Thus, sodium homeostasis is essential, as reported by Zhou and Liao.11 High sodium concentrations can cause changes in blood vessels, primarily by acting on the endothelium.
Cells originating from the mesoderm have a one-way sodium transport direction, causing an increase in interstitial sodium concentration with an active transport mechanism. At the capillary site, salt is exchanged through diffusion on a concentration gradient, and there should be a sodium circulation mode in the body. If the sodium level is higher in intercellular space than in intracellular space and plasma, sodium travels from a high-concentration space to a low-concentration space through diffusion. Thus far, lipid transmission through the endothelium is not understood, and it is thought that macrophages engulf denatured protein as a defence mechanism. Further studies are needed to confirm this hypothesis. Meanwhile, more stringent control of sodium intake by those who are at a higher risk of developing IHD is encouraged.
Given the evidence on the unfavourable association between salt intake and IHD, Sri Lankan policy planners are disseminating the message of limiting the daily intake of salt to a maximum of 5g per adult. This analysis confirmed that an increased sodium intake of even 1g of salt above the recommended level would significantly contribute to a high level of IHD, indicated by the formation of >5 segmental lesions.
Study Limitations
Dietary consumption was based on details reported by participants. The limitations associated with this method have been previously outlined.21 However, several measures were taken to ensure that reliable responses were elicited. Although the association between salt intake and atheroma formation was adjusted for seven confounders, more known or unknown confounders could have affected this association. This should also be considered when interpreting the findings.
Sodium levels in urine samples must be measured, but since reliable objective measurement requires 24-hour urine collections this is impracticable in clinical settings and there is no standard way to monitor sodium consumption in individuals.33 Taking into consideration the cost of this process, this was not performed. Thus, the calculation of the sodium level was based on responses to questionnaires. Using different techniques to measure plaque volumes would have given more accurate information.
Conclusion
When calculated based on the intake of table salt, the daily intake of sodium appears to increase the risk of atheroma formation in more coronary segments irrespective of age, sex, several known comorbidities and risk factors. Therefore, the authors encourage the public to pay more attention to regulating salt consumption to avoid atheroma formation and minimise risk factors.
Click here to view Supplementary Material
Clinical Perspective
- This cross-sectional study evaluated the influence of salt intake on segmental lesions in patients with ischaemic heart disease in Sri Lanka.
- The average intake of sodium per day per person was 6,176 mg/day in the sample, despite the WHO-recommended level of 2,000 mg/day and the WHO-recommended upper limit of sodium intake was exceeded by all participants.
- The daily intake of sodium positively correlated with atheroma formation in more coronary artery segments.