The use of high-sensitivity cardiac troponin (hs-cTn) in the emergency department is adopted as the standard of care in many parts of the world. The higher sensitivity and analytical precision at the lower concentrations improved the ability of hs-cTn to accurately detect minor changes in troponin concentrations associated with myocardial injury, including ischaemic events.1 This has led to changes in the universal definition of MI to include evidence of elevation of cTn values with at least one value above the 99th percentile upper reference limit, as well as the detection of kinetic changes in cTn concentrations as a rise and/or fall of cTn values.2
Elevated hs-cTn values suggest injury to the myocardial cells, although this does not indicate the underlying cause of injury; thus, the differential diagnosis of elevated hs-cTn includes a huge list of ischaemic and non-ischaemic causes of cardiac injury.1
Several multicentre clinical trials examined the usage of hs-cTn for the accelerated triaging of patients presenting to the emergency department (ED) with chest pain and particularly in the absence of conclusive ECG changes, mainly non-ST-elevation MI (NSTEMI). This has led to the incorporation of hs-cTn in various guidelines and consensus group recommendations, including the European Society of Cardiology (ESC) and the Asian Pacific Society of Cardiology (APSC).3,4 Both guidelines proposed algorithms for the rapid rule-in and rule-out of acute MI patients presenting to the ED with NSTEMI within 2–6 hours of symptoms.3,4
Shariff et al. reported that the Malaysian Expert Consensus Group are proposing a national guideline on the use of high-sensitivity troponins in the ED in Malaysia.5 The group recommended the use of a sex-specific 99th percentile upper reference limit (URL) as the basis to classify patients as ‘negative’ or ‘positive’ for hs-cTn, applying the History, Electrocardiogram, Age, Risk factors and Troponin risk score for the negative patients to decide on the final disposition of discharge or admission with repeat testing in the high-risk group. The absolute reference change value (RCV) in ng/l for the assay is recommended by the group to be used to determine whether the difference between the 0- and 2-hour values is significant. The use of the 0-hour/3-hour algorithm is recommended if retesting within 2 hours is a challenge.
The ESC and APSC guidelines recommend 0-hour/1-hour, 0-hour/2-hour and 0-hour/3-hour algorithms based on the limit of detection of the assay, the 99th percentile URL, the magnitude of increase and the delta changes in serial measurements to categorise patients with suspected NSTEMI into rule-in, rule-out or observe categories with suggested final dispositions to either discharge, admit to the ward or admit to the cardiac observation unit.3,4
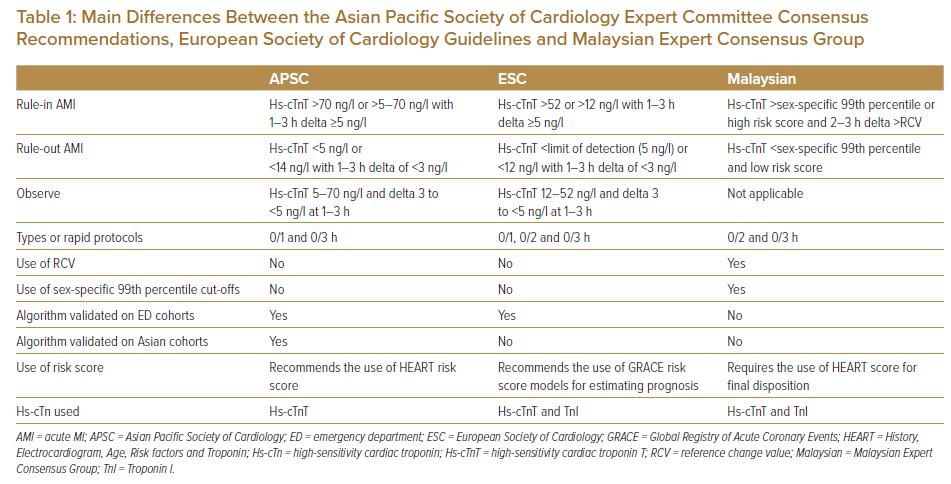
Shiozaki et al. examined the APSC and ESC algorithms performances in a prospective study of the ESC 0-hour/1-hour algorithm in Japan and Taiwan.6,7 Using the cohort data, 903 patients with a prevalence rate of acute MI of 13.1%, the utility of the ESC 0-hour/1-hour algorithm and the APSC algorithm were compared. Major adverse cardiovascular events consisted of all-cause mortality, subsequent acute MI and unexpected coronary revascularisation in 30 days. The study excluded ST-elevation MI and chronic kidney disease, defined as >3.0 mg/dl of serum creatinine level. The distribution pattern of the stratification was similar (ESC versus APSC; rule-out 23% versus 22%, observation 34% versus 33%, rule-in 43% versus 45%); however, with lower sensitivity compared with the ESC algorithm in this population. The sensitivity was 99.9% (95% CI [94.0–99.9]) versus 95.7% (95% CI [89.2–98.8]) and specificity 76.6% (95% CI [72.8–80.2]) versus 78.3% (95% CI [74.5–81.7]), respectively (unpublished data). Table 1 summarises the main differences between the APSC Expert Committee consensus recommendations, ESC guidelines and Malaysian Expert Consensus Group in the use of high-sensitivity cardiac troponin T (hs-cTnT).
As the global adoption of hs-cTn continues, the paper by the Malaysian Expert Consensus Group provides yet another different perspective for the application of the rapid rule-in and rule-out algorithms using hs-cTn in the ED setting to triage patients with acute coronary syndrome.5
National guidelines unify the practice recommendation for the country and decrease variations between hospitals considering the countries’ own disease prevalence and practice limitations. This also facilitates and streamlines the transfer of patients between various medical institutions within the country. The Malaysian Expert Census Group is to be congratulated on their effort to drive for standardisation of practice in ED triaging patients with suspected acute coronary syndrome across Malaysia.
One caveat in the proposed guideline is the use of the 99th percentile URL as ‘positive’ or ‘negative’, as this may lead to increased uptake due to chronic elevations of hs-cTn in some conditions, such as chronic renal failure. This dichotomous classification does not apply only to hs-cTn assays, and detection of kinetic changes is required to enhance test performance. Another point to highlight is the use of the RCV as an indicator of a significant change in hs-cTn concentrations. Decision thresholds include RCV of >20% from a baseline value >99th percentile URL; and a RCV >50% from a baseline value ≤99th percentile URL. If we would apply the proposed RCV for hs-cTnT assay with an overall 99th percentile URL of 19 ng/l, this would translate into >9.5 ng/l from a baseline value ≤99th percentile URL, which is different to the 3–5 ng/l delta proposed by the APSC consensus recommendation and the ESC.3,4
RCV incorporates both biological variation as well as analytical variations in determining whether the change in the serial concentrations is significant or not. Although this is a very valid analytical argument, this approach has not been validated in clinical studies, and the values are different to the delta changes recommended by the ESC and APSC guidelines, which were tested and validated in several prospective clinical studies. The RCV for hs-cTnT is calculated to be between 20% and 44% by various authors using various platforms to measure hs-cTnT.8 In addition, biological variations will vary in individuals with chronic kidney diseases compared with the healthy individuals from which most of the data on biological variation are derived.
The use of age-specific 99th percentile thresholds is another difference between the Malaysian group recommendations and the APSC and ESC guidelines. The use of age-specific cut-offs is not required in the APSC and ESC guidelines, as they both use the limit of detection of the assay as the basis for rule-out, which is much lower than the 99th percentile reference limit, and this was validated prospectively on mixed cohorts.
Regarding the incorporation of the History, Electrocardiogram, Age, Risk factors and Troponin score for risk stratification, although it is a good recommendation and especially for those with ‘negative’ work-up to better identify patients at increased risk of cardiovascular events, it may not add much to the classification of patients based on hs-cTn and clinical judgement. In a comprehensive prospective study of the performance of chest pain scores in the ED in Singapore, although the History, Electrocardiogram, Age, Risk factors and Troponin score had the highest sensitivity of 88.1% (95% CI [81.5–92.6]) among the chest pain scores, it was extremely comparable with clinical judgement, at 85.5% sensitivity (95% CI [78.3–90.6]), with an overall area under curve value of 0.794.9
Similar to the APSC, the use of point-of-care cardiac troponin assays in the ED was not encouraged due to the lower sensitivity and negative predictive values of those point-of-care testing assays compared with hs-cTn assays, although there are newer devices in the pipeline that may have promising analytical sensitivity and performance.10
In summary, the paper from the Malaysian Expert Census Group provides a modified approach to the APSC and ESC for triaging patients with acute coronary syndrome for the ED in Malaysia.5 The proposed algorithm remains to be validated in a large population and its clinical performance compared with the algorithm proposed by other guidelines. The APSC guidelines, thus, remain the most recommended guidelines to follow in the Asia-Pacific region when using hs-cTnT, followed by the ESC when using high-sensitivity cardiac troponin I.
National guidelines unify the practice recommendation for the country and decrease variations between hospitals considering the countries’ own disease prevalence and practice limitations. This also facilitates and streamlines the transfer of patients between various medical institutions within the country. The Malaysian Expert Census Group is to be congratulated on their effort to drive for standardisation of practice in ED triaging patients with suspected acute coronary syndrome across Malaysia.
One caveat in the proposed guideline is the use of the 99th percentile URL as ‘positive’ or ‘negative’, as this may lead to increased uptake due to chronic elevations of hs-cTn in some conditions, such as chronic renal failure. This dichotomous classification does not apply only to hs-cTn assays, and detection of kinetic changes is required to enhance test performance. Another point to highlight is the use of the RCV as an indicator of a significant change in hs-cTn concentrations. Decision thresholds include RCV of >20% from a baseline value >99th percentile URL; and a RCV >50% from a baseline value ≤99th percentile URL. If we would apply the proposed RCV for hs-cTnT assay with an overall 99th percentile URL of 19 ng/l, this would translate into >9.5 ng/l from a baseline value ≤99th percentile URL, which is different to the 3–5 ng/l delta proposed by the APSC consensus recommendation and the ESC.3,4
RCV incorporates both biological variation as well as analytical variations in determining whether the change in the serial concentrations is significant or not. Although this is a very valid analytical argument, this approach has not been validated in clinical studies, and the values are different to the delta changes recommended by the ESC and APSC guidelines, which were tested and validated in several prospective clinical studies. The RCV for hs-cTnT is calculated to be between 20% and 44% by various authors using various platforms to measure hs-cTnT.8 In addition, biological variations will vary in individuals with chronic kidney diseases compared with the healthy individuals from which most of the data on biological variation are derived.
The use of age-specific 99th percentile thresholds is another difference between the Malaysian group recommendations and the APSC and ESC guidelines. The use of age-specific cut-offs is not required in the APSC and ESC guidelines, as they both use the limit of detection of the assay as the basis for rule-out, which is much lower than the 99th percentile reference limit, and this was validated prospectively on mixed cohorts.
Regarding the incorporation of the History, Electrocardiogram, Age, Risk factors and Troponin score for risk stratification, although it is a good recommendation and especially for those with ‘negative’ work-up to better identify patients at increased risk of cardiovascular events, it may not add much to the classification of patients based on hs-cTn and clinical judgement. In a comprehensive prospective study of the performance of chest pain scores in the ED in Singapore, although the History, Electrocardiogram, Age, Risk factors and Troponin score had the highest sensitivity of 88.1% (95% CI [81.5–92.6]) among the chest pain scores, it was extremely comparable with clinical judgement, at 85.5% sensitivity (95% CI [78.3–90.6]), with an overall area under curve value of 0.794.9
Similar to the APSC, the use of point-of-care cardiac troponin assays in the ED was not encouraged due to the lower sensitivity and negative predictive values of those point-of-care testing assays compared with hs-cTn assays, although there are newer devices in the pipeline that may have promising analytical sensitivity and performance.10
In summary, the paper from the Malaysian Expert Census Group provides a modified approach to the APSC and ESC for triaging patients with acute coronary syndrome for the ED in Malaysia.5 The proposed algorithm remains to be validated in a large population and its clinical performance compared with the algorithm proposed by other guidelines. The APSC guidelines, thus, remain the most recommended guidelines to follow in the Asia-Pacific region when using hs-cTnT, followed by the ESC when using high-sensitivity cardiac troponin I.









