Acute coronary syndrome (ACS) occurs when an atherosclerotic plaque disrupts the flow of blood, resulting in activation of platelets and coagulation factors and leading to the formation of a thrombus. Depending upon the degree and acuteness of thrombus formation and coronary occlusion, the patient can present with non-ST-elevation ACS or ST-elevation MI (STEMI). Non-ST-elevation ACS is further classified as non-ST-elevation MI (NSTEMI) or unstable angina (UA). In NSTEMI there is myocardial injury and the cardiac biomarkers are elevated, whereas in UA the cardiac biomarkers are normal.
Dual antiplatelet therapy (DAPT) with aspirin plus a P2Y12 inhibitor is the cornerstone of treatment after percutaneous coronary intervention (PCI) for ACS. DAPT has a twofold benefit in these patients. First, DAPT is crucial to reduce the risk of stent thrombosis, MI and target lesion revascularisation at the PCI coronary site. Second, patients with ACS are at high risk of recurrent ischaemic events at other sites, which can be prevented by DAPT. European and American guidelines recommend the use of DAPT for 12 months after ACS and PCI, with the highest level of evidence.1,2
While 1-year DAPT has been recommended with first-generation drug-eluting stents (DES) to prevent late stent thrombosis, with newer-generation DES the duration of DAPT can be significantly reduced.3,4 Bleeding risk increases in line with DAPT duration regardless of which P2Y12 inhibitor is used.5 Major bleeding in patients receiving DAPT has an impact on mortality after ACS that can be greater than that of recurrent MI.6
There is emerging evidence that shorter-duration DAPT with the newer-generation DES has similar efficacy and safety to the gold standard DAPT (1 year) in the setting of ACS and PCI.7–10
The COMBO stent (OrbusNeich Medical) was used in the REDUCE (Short-term Dual Antiplatelet Therapy in Patients with ACS Treated with the COMBO Dual-therapy Stent) trial.11 COMBO is a dual-therapy stent with abluminal sirolimus elution from a biodegradable polymer and a luminal pro-healing anti-CD34+ antibody layer, which attracts circulating endothelial progenitor cells to provide rapid endothelialisation. This novel technology may allow for a shorter duration of DAPT after PCI in the setting of ACS. The objective of this analysis was to evaluate differences between NSTEMI/UA and STEMI patients from the REDUCE trial population in terms of their characteristics, procedures and outcomes.
Methods
REDUCE (NCT02118870) was an investigator-initiated prospective, multicentre trial that randomised ACS patients after treatment with the COMBO stent to either 3 or 12 months of DAPT.12 The study was conducted at 36 investigational sites in Europe and Asia and was designed to enrol 1,500 ACS patients. The study design and randomisation, ethics approval, treatment and follow-up procedures, study endpoints, statistical consideration and results were published in 2019.11 The primary endpoints were all-cause mortality, MI, stent thrombosis, stroke, target vessel revascularisation (TVR) and bleeding (Bleeding Academic Research Consortium [BARC] II, III and V). Of the 1,500 eligible ACS patients undergoing successful COMBO stent implantation, four patients revoked their informed consent soon after randomisation, leaving 1,496 patients for analysis. Of these 1,496 patients, 789 (52.8%) had NSTEMI/UA and 706 (47.2%) had STEMI. In the NSTEMI/UA cohort when 3- and 12-month DAPT were compared, the only significant differences were for body weight (mean 80.97 ± 15.81 kg versus 78.88 ± 14.85 kg; p=0.0487) and spironolactone use (3.2% versus 1.0%; p=0.037), both of which were higher in the 3-month group. In the STEMI cohort, the 3-month group had a higher rate of previous PCI than the 12-month group (7.0% versus 3.6%; p=0.0422). The patient profiles were otherwise similar. Thus, in this current analysis, 789 NSTEMI/UA patients were compared with 706 STEMI patients in terms of patient characteristics, PCI procedures and outcomes.
Statistical Analysis
Cumulative event rates were estimated using the Kaplan–Meier method and compared using the log-rank test. The incidence of the events was also compared using the χ2 test or Fisher’s exact test. Normally distributed variables were compared using the Student’s t-test. Otherwise, the Mann–Whitney U-test was used. Categorical variables are summarised in terms of number and percentages and were compared using the two-sided χ2 test or Fisher’s exact test when applicable; p≤0.05 was considered statistically significant.
Results
The overall number of patients in the NSTEMI/UA cohort (789; 52.8%) was compared with the overall number of STEMI cohort patients (706; 47.2%). In this subgroup analysis of the REDUCE trial for the NSTEMI/UA and STEMI cohorts, comparing 3-month versus 12-month DAPT, there were no statistically significant differences in the primary endpoints at 360 days and at 720 days between the two cohorts. However, in the NSTEMI/UA cohort, 3-month DAPT was better than 12-month DAPT regarding the primary endpoint outcomes (9.6% versus 10.0% at 360 days and 12.1% versus 15.0% at 720 days). In contrast, for the STEMI cohort, primary endpoint outcomes for 12-month DAPT were better than for 3-month DAPT (3.3% versus 4.1% at 180 days, 6.6% versus 6.6% at 360 days, 8.4% versus 11.0% at 720 days).
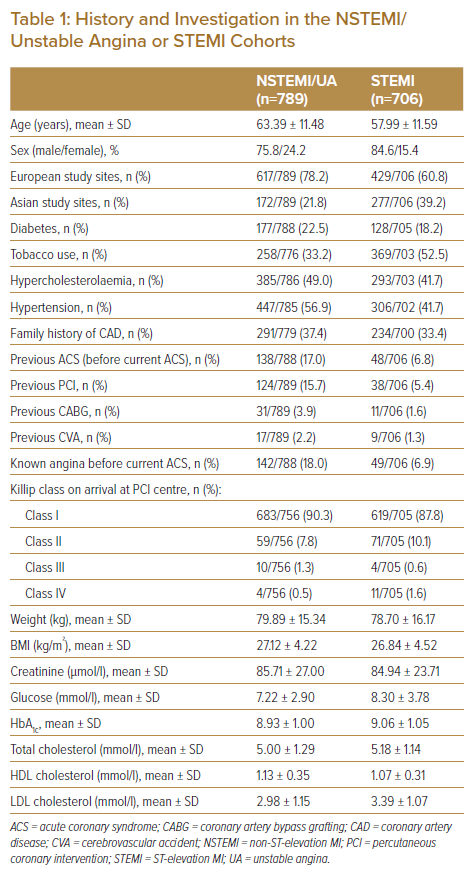
Patients from the NSTEMI/UA cohort were older (mean age, 63.39 ± 11.48 years versus 57.99 ± 11.59 years) and more of them were women (24.2% versus 15.4%) than in the STEMI cohort. In the NSTEMI/UA cohort, patient recruitment was higher at the European sites than Asian sites (78.2% versus 60.8%). They had higher rates of conventional cardiovascular risk factors than the STEMI cohort (diabetes, 22.5% versus 18.2%; hypercholesterolaemia, 49.0% versus 41.7%; and hypertension, 56.9% versus 41.5%). Tobacco use was higher in the STEMI cohort than in the NSTEMI/UA cohort (52.5% versus 33.2%). NSTEMI/UA patients had a higher rate of family history of coronary artery disease than STEMI patients (37.4% versus 33.4%) and higher rates of previous ACS (17.0% versus 6.8%) and known angina (18.0% versus 6.9%) prior to the current event. They also had higher rates of previous PCI (15.7% versus 5.4%), previous coronary artery bypass graft surgery (3.9% versus 1.6%) and previous stroke (2.2% versus 1.3%) than the STEMI cohort. NSTEMI/UA patients had higher body weight than STEMI patients (mean ± SD: 79.89 ± 15.34 kg versus 78.70 ± 16.17 kg) and had a higher mean BMI. Their mean creatinine level was higher (85.71 ± 27.00 µmol/l versus 84.94 ± 23.71 µmol/l). However, mean glucose, mean HbA1c, total cholesterol and LDL cholesterol were lower in the NSTEMI/UA cohort than the STEMI cohort (Table 1).
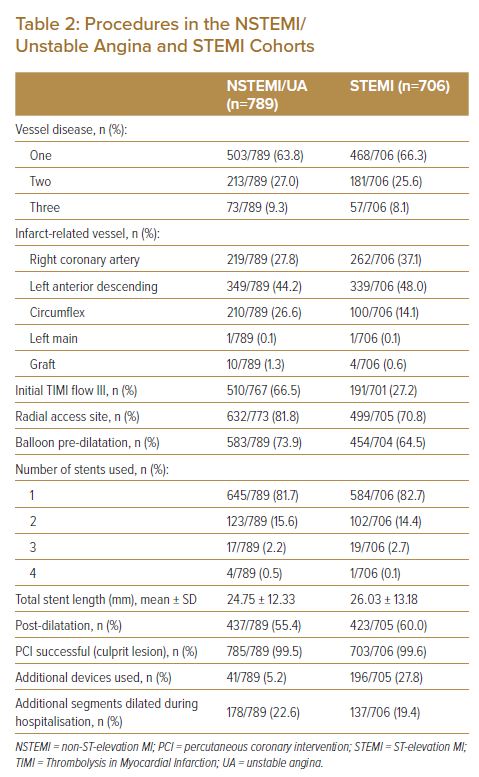
In the NSTEMI/UA cohort, more procedures were performed using radial site access (81.8% versus 70.8%) and this cohort had a higher rate of initial thrombolysis in MI (TIMI) III flow (66.5% versus 27.2%) than the STEMI cohort. They also had higher balloon pre-dilatation (73.9% versus 64.5%). However, post-balloon dilation was similar (55.4% versus 60.0%). PCI success rates were also similar between the two cohorts (almost 100%), along with the number and length of stents used. Thrombosuction devices were more frequently used in the STEMI cohort than in the NSTEMI/UA cohort (27.8% versus 5.2%; Table 2).
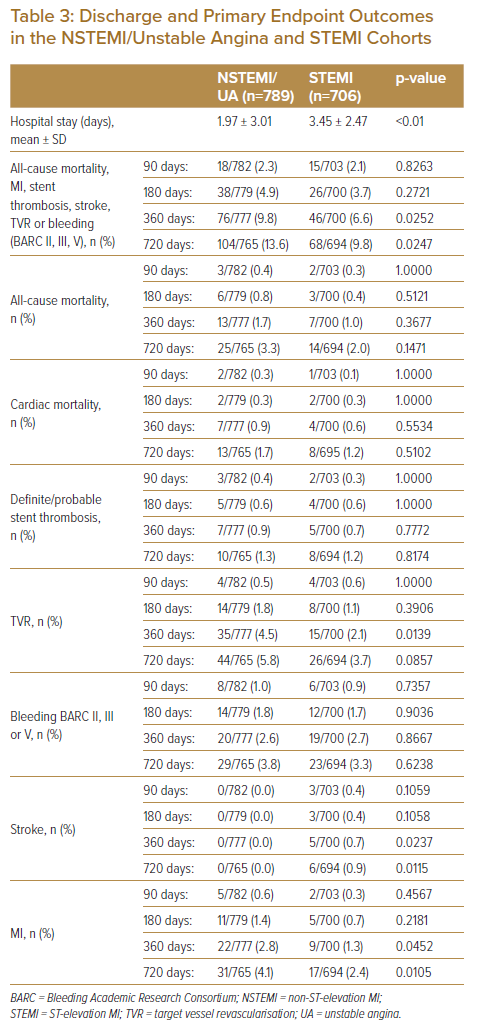
Length of hospital stay was longer in the STEMI cohort than in the NSTEMI/UA cohort (mean ± SD: 3.45 ± 2.47 days versus 1.97 ± 3.01 days). Discharge medications were similar except for the calcium antagonists, which were prescribed at higher rates in the NSTEMI/UA cohort than in the STEMI cohort (17.3% versus 6.8%; Table 3).
Outcomes
The number of patients from the overall NSTEMI/UA and STEMI cohorts available for outcome analysis at various time intervals is as follows: 782 versus 703 at 90 days; 779 versus 700 at 180 days; 777 versus 700 at 360 days; and 765 versus 694 at 720 days.
When comparing the overall NSTEMI/UA cohort with the overall STEMI cohort for the primary endpoint indices of all-cause mortality, MI, stent thrombosis, stroke, TVR and bleeding (BARC II, III, V), rates in the NSTEMI/UA cohort were consistently higher than in the STEMI cohort: 2.3% versus 2.1% at 90 days (p=0.8262); 4.9% versus 3.7% at 180 days (p=0.2721); 9.8% versus 6.6% at 360 days (p=0.0252); and 13.6% versus 9.8% at 720 days (p=0.0247). This was attributed to higher all-cause mortality at 180 days (0.8% versus 0.4%), 360 days (1.7% versus 1.0%) and 720 days (3.3% versus 2.0%); higher TVR at 180 days (1.8% versus 1.1%), 360 days (4.5% versus 2.1%) and 720 days (5.8% versus 3.7%); and higher MI at 180 days (1.4% versus 0.7%), 360 days (2.8% versus 1.3%) and 720 days (4.1% versus 2.4%). Revascularisation within 360 and 720 days was also higher in the NSTEMI/UA cohort than in the STEMI cohort (7.6% versus 3.7% and 10.8% versus 6.1%, respectively).
There were no differences in cardiac mortality and bleeding (BARC II, III and V) between the two cohorts. Despite these being ACS patients undergoing PCI, the incidence of stent thrombosis was similarly very low in the two cohorts (Table 3).
Discussion
The REDUCE trial shows that in ACS patients treated with the COMBO stent, 3-month DAPT is non-inferior to 12-month DAPT regarding the primary endpoint indices of all-cause mortality, MI, stent thrombosis, stroke, TVR and bleeding (BARC II, III and V). Similar outcomes between the two groups were observed at 2-year follow-up (11.6% versus 12.1%).11 In the REDUCE trial population, of the 1,496 patients eligible for analysis, 789 (52.8%) were in the NSTEMI/UA cohort and 706 (47.2%) were in the STEMI cohort. This subgroup analysis of NSTEMI/UA and STEMI patients therefore provides a unique opportunity to compare the differences in terms of clinical characteristics, PCI procedures and outcomes between the two cohorts in the era of the new-generation DES in the setting of ACS and contemporary practice.
Although NSTEMI/UA and STEMI share many similarities in terms of risk factors, demographics and pathogenesis of plaque rupture, they are two distinct pathophysiological entities. STEMI is usually characterised by severe and/or total coronary flow obstruction with transmural ischaemia, which predisposes to myocardial necrosis and pump dysfunction. The pathogenesis of NSTEMI differs from that of STEMI in that it usually results from a flow-limiting coronary stenosis with resultant downstream myocardial ischaemia. However, total coronary artery occlusion does exist in a significant number of patients presenting with NSTEMI/UA, thus this analysis will provide insights into the DAPT strategy between NSTEMI/UA and STEMI in the era of the new-generation DES.
In this subgroup analysis of the REDUCE trial in the NSTEMI/UA cohort and STEMI cohort comparing 3-month DAPT versus 12-month DAPT, the primary endpoint indices at 360 and 720 days were not statistically significantly different.11 However, in the NSTEMI/UA cohort, 3-month DAPT appears to be safe in terms of 360- and 720-day outcomes. In contrast, for the STEMI cohort, 12-month DAPT appears to be better for the 720-day outcomes.
With the introduction of newer generation DES, the minimum duration of DAPT recommended by the guidelines has been reduced to 6 months in patients with stable coronary artery disease.1,2 Whether a shorter duration of DAPT is safe in patients presenting with ACS in the contemporary era remains controversial. Furthermore, limited data exist comparing the duration of DAPT between NSTEMI/UA and STEMI patients.
In a network meta-analysis by Yin et al. that included 17 studies with 46,864 patients, eight trials reported endpoints in ACS subgroups (12,376 patients).10 In the subgroup analysis of patients with ACS, short-duration DAPT (3–6 months) showed similar efficacy and safety to standard 12-month DAPT.
Verdoia et al. conducted a meta-analysis of 17,941 ACS patients undergoing PCI, which also showed that a shorter duration of DAPT (3–6 months) may be safely considered.13 The authors reported similar rates of recurrent thrombotic complications and mortality compared with the standard 12-month DAPT and a significant reduction in major bleeding complications.
In a recent network meta-analysis of randomised trials by Kuno et al., 14 eligible trials enrolling a total of 31,837 patients comparing different DAPT durations (≤3, 6, 12 and >12 months) in ACS patients undergoing PCI were examined. The main findings were that short-term DAPT (≤3 or 6 months) did not increase ischaemic outcomes compared with long-term DAPT (12 and >12 months).14 For bleeding outcomes, ≤3-month DAPT was associated with significant reduction in bleeding compared with 6-, 12- or >12-month DAPT.
Data from these meta-analyses of randomised trials support short-term (≤3- and 6-month) DAPT, even in patients with ACS undergoing PCI using the newer generation DES. However, there is a paucity of data comparing the different durations of DAPT in NSTEMI/UA and STEMI patients. Our analysis suggests that 3 months of DAPT for NSTEMI/UA patients results in numerically lower outcomes of the primary endpoints.
However, for STEMI patients, the 12-month DAPT outcomes may be numerically better. This may be explained by the higher thrombotic risk in STEMI, which requires a longer DAPT duration than in NSTEMI/UA. Moreover, in patients with STEMI, the daily risk of ischaemia significantly exceeded the daily risk of bleeding beyond 30 days, supporting the use of a longer duration of DAPT after STEMI.15
Patients from the NSTEMI/UA cohort were older, were more often women and had higher rates of cardiovascular risk factors (diabetes, hypercholesterolaemia and hypertension). Tobacco use was higher in the STEMI cohort. NSTEMI/UA patients had higher rates of previous ACS, known angina, previous stroke and a higher mean creatinine level. Length of hospital stay was longer in the STEMI cohort than in the NSTEMI/UA cohort. Discharged medications were similar except for calcium antagonists, which were prescribed more frequently in the NSTEMI/UA cohort than in the STEMI cohort. These findings are similar to the Malaysian Annual Report of the NCVD-ACS Registry, 2014–15.16 Similar findings were also reported in the Second Euro Heart Survey on ACS.17
Patients presenting with STEMI are known to have a higher risk of early mortality, but patients with NSTEMI/UA have been shown to have a higher risk of long-term mortality, attributed to a higher burden of comorbidities and multivessel disease.18–20
In our analysis, the NSTEMI/UA cohort appeared to have higher rates of primary endpoint indices than the STEMI cohort at 90, 180, 360 and 720 days. This was attributed to higher all-cause mortality, TVR and MI. Data from the ACTION Registry–Get With The Guidelines indicated that STEMI patients had significantly lower comorbidities than NSTEMI patients.21 They were more likely to present with a larger infarct and had higher predicted in-hospital mortality. However, the unadjusted cumulative incidence of all-cause mortality and a composite outcome including mortality and non-fatal cardiovascular and cerebrovascular outcomes, was lower for STEMI patients from hospital discharge through 2 years.21 Similar findings were noted from the Malaysian NCVD-ACS egistry.16 Outcomes for STEMI compared with NSTEMI for in-hospital mortality were 10.6% versus 8.0%, whereas at 1 year they were 17.9% versus 23.0%.16
Study Limitations
This study was a subgroup analysis with all the inherent limitations in the interpretation of data. The REDUCE trial was adequately powered. However, in this subgroup analysis, comparing NSTEMI/UA versus STEMI cohorts, the sample size was not adequately powered to determine the difference in outcomes between the two cohorts for 3- and 12-month DAPT. Furthermore, for simplicity of comparison, the overall patients in the NSTEMI/UA and STEMI cohorts were compared to determine the difference between patient profile and outcomes, although there were some minor differences between the two cohorts.
Conclusion
In the REDUCE trial population, a numerically lower occurrence of outcomes was observed with the shorter duration of DAPT for NSTEMI patients, whereas for STEMI patients, 12-month DAPT appeared to be better. There is no statistical interaction across the subgroups and this observation is exploratory. These findings will need to be tested in a randomised controlled trial with adequate sample size. In this analysis, the overall NSTEMI/UA cohort tended to have significantly higher primary endpoint indices at 360 and 720 days. This highlights the importance of risk assessment tools to identify high-risk patients, early PCI and good guideline-directed medical therapy to further improve long-term outcomes in the NSTEMI/UA population.
Clinical Perspective
- There is a paucity of data on the duration of dual antiplatelet therapy (DAPT) comparing non-ST-elevation MI (NSTEMI) and ST-elevation MI (STEMI) in contemporary percutaneous coronary intervention in the era of new-generation drug-eluting stents.
- Our analysis suggests that shorter DAPT may be suitable for NSTEMI patients and that longer DAPT may be suitable for STEMI patients.
- These findings need to be further tested in a randomised controlled study.