Asia, which comprises one-third of the world’s land area, is densely populated and is the home of two-thirds of the global population.1 Competition for access to limited resources typically exists in many developing countries in this region. Healthcare services in many Asian countries are either fully or partially government-funded via the public insurance system.2 Stroke is a major cause of morbidity and mortality worldwide and was once considered a highly prevalent disease in developed countries. The burden of stroke has drastically decreased in developed countries through the application of evidence-based practices. In contrast, the burden of stroke seems to be increasing in developing countries.3 In Asia the burden of stroke is much higher in the north, and this burden is likely to increase substantially in the next few decades in other developing countries due to the ageing population and health transition as a result of urbanisation and rapid transformation.4,5
A large proportion of thromboembolic stroke is associated with AF.6,7 Stroke related to AF is frequently devastating and is associated with a high recurrence rate of more disabling secondary events.8,9 Although stroke due to AF is common, 25–40% of AF cases are paroxysmal and clinically silent; hence, the detection of AF in patients with ischaemic stroke, particularly those who do not present with AF on admission, can be a major challenge.10 Prolonged cardiac monitoring is a recommended strategy for increasing the detection rate of AF in patients with ischaemic stroke and transient ischaemic attack (TIA).11,12 This review paper discusses screening for AF with a focus on patients admitted for ischaemic stroke and TIA from the perspective of the Asian population, in which the onset of thromboembolic stroke tends to occur at a younger age despite evident geographical, ethnic, age and gender variances.13–17
Search Strategy and Selection Criteria
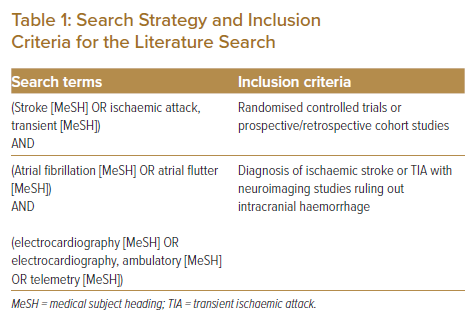
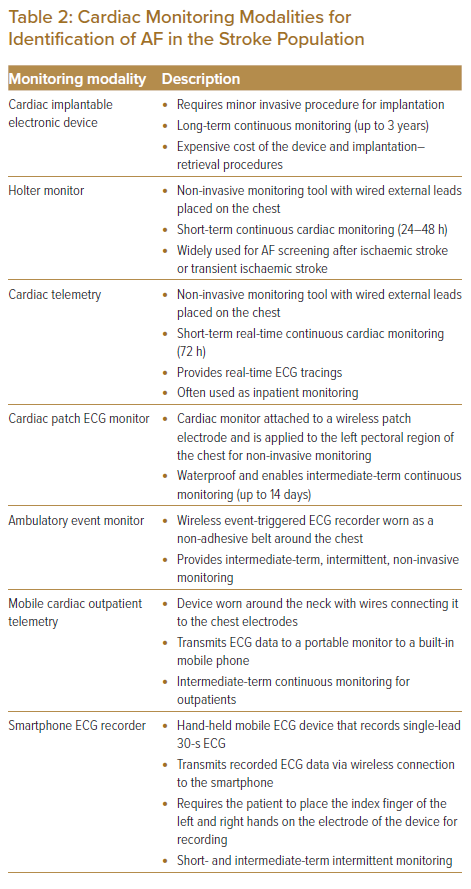
In this non-systematic review paper, we searched all studies published between 1990 and 30 October 2021 and which were listed in the PubMed database. Search terms are listed in Table 1. Inclusion criteria included: randomised controlled trials or prospective/retrospective cohort studies; and patients with a diagnosis of ischaemic stroke or TIA with neuroimaging ruling out intracranial haemorrhage. Studies reporting duplicated cohorts, abstracts from studies presented at scientific meetings but not published as full manuscripts, publications with content redundancy, and publications with no description of the methods were excluded. Table 2 summarises cardiac monitoring modalities for the identification of AF in the stroke population that will be further discussed in this paper.
Characteristics of Trials and Study Population in Asia
Various studies have been conducted for more than a decade to investigate screening strategies for the detection of AF in patients with ischaemic stroke. However, representation of the Asian population (two-thirds of the world population) is lacking in many large international trials.18,19 Methods of detection of occult AF for secondary stroke prevention have not been widely investigated, most probably due to the perception of the lower prevalence of AF in Asia.20−31 However, considering the burden of AF and stroke in the Asian population, more studies have been conducted to address the knowledge and practice gaps in AF screening by targeting patients with stroke in the past 5–6 years (Supplementary Material Table 1). Of these studies, seven were prospective observational studies, two were randomised controlled trials, and two were retrospective observational studies. More than half of the studies included patients with both ischaemic stroke and TIA, while the remaining studies involved patients with only ischaemic stroke.
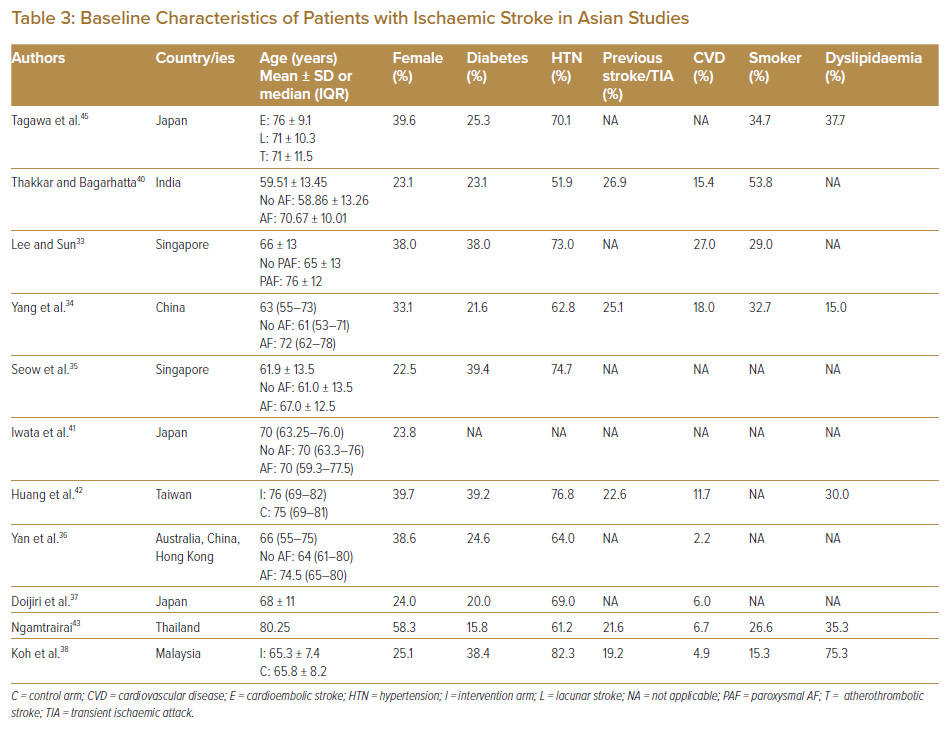
Compared with the study cohort in the landmark CRYSTAL-AF trial, which represents the Western population in Europe, the US and Canada, the mean and median ages of the Asian cohorts studied were mostly similar.32−38 It is known, based on evidence from the European population, that the risk of death due to AF increases exponentially in patients above 60 years of age, and some of these Asian trials reported that AF or paroxysmal AF affected patients with ischaemic stroke of similar age (70–76 years).33,34,36,39–41 Questions, however, remain on whether age should be set as a limit (as in some Asian trials listed in Supplementary Material Table 1) for the inclusion of patients in the trials, given that Asian patients with ischaemic stroke are younger.13–17
The rate of stroke recurrence was considerably high, with a reported prevalence of previous stroke or TIA of between 19.2% and 26.9% (Table 3).34,38,40,42,43 Compared with the risk profiles in the GARFIELD-AF registry, which enrolled 10,614 patients with newly diagnosed AF at risk of stroke in the Asia-Pacific region, Europe and Central and South America, the prevalence of diabetes varied from 15.8% to 39.4% (versus 22.0%) across these studies.33–38,40,42–45 Interestingly, one study from Malaysia reported the prevalence of dyslipidaemia to be as high as 75.3%, and another study on the Indian population reported that the prevalence of smoking was the highest at 53.8%, even compared with that of hypertension (51.9%).38,40
Comparison of Findings in Asian and Western Trials
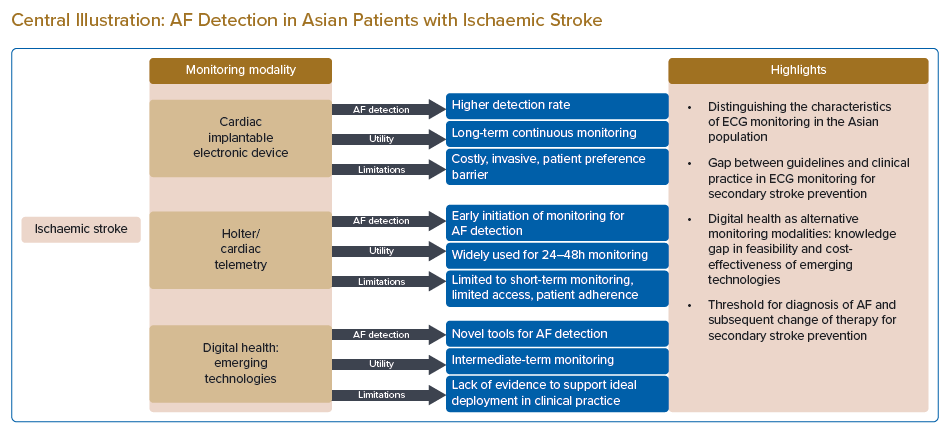
In the Asian population, cardiac implantable electronic devices (CIEDs) undoubtedly detect more cases of AF (15.5%–27%) than non-invasive cardiac monitoring strategies in patients with ischaemic stroke regardless of the timing of initiation (Supplementary Material Table 2).35,37,41 Iwata et al. and Doijiri et al. reported AF detection rates of 26.2% and 27.0%, respectively, at a median duration of 7 months in Japan; and Seow et al. reported a detection rate of 15.5% at 3 years for the Singaporean population.35,37,41 For non-invasive cardiac monitoring, many Asian studies initiated intensified AF screening early (from less than 24 hours after index stroke events to within 7 days after the onset of stroke) with Holter or continuous ECG monitoring in patients who were admitted for ischaemic stroke, and reported AF detection rates ranging from 8% to 9.35%.33,34,43,45 Two studies that evaluated the detection of AF in patients with ischaemic stroke and TIA using intermittent mobile ECG recording with KardiaMobile (AliveCor) reported similar AF detection rates, although one study reported early initiation of intermittent mobile ECG recording (within 24 hours of onset of the event) and continued monitoring until the patients were discharged from the hospitals (median 4-day duration of monitoring), while the other study initiated monitoring late (within 12 months of the onset of stroke) and continued for 30 days.36,38 Based on this evidence, early initiation of intensified AF screening could be considered advantageous in the Asian population.46
In the Western population, it was observed that the non-invasive cardiac monitoring strategies, if initiated early (in the first week after the onset of stroke), had comparable diagnostic yield (12.5–13.5%) to the CIEDs, despite a shorter monitoring period.47−49 Thus, the benefits of monitoring with CIEDs will become evident only with long-term monitoring (beyond 12 months). Data from the landmark CRYSTAL-AF trial, for example, established that the detection rate associated with CIEDs was 12.4% at 12 months, which increased to 30% at 36 months.32,50 Ziegler et al. reported detection rates of 12.2%, 16.3% and 21.5% at 6, 12 and 24 months, respectively.47
The results from these Asian and Western trials are not directly comparable and therefore cannot be used to establish ethnic differences in AF detection rates between Asian patients and their Western counterparts, or even among the multi-ethnic Asian population. Future study designs and meta-analyses could perhaps consider such an ethnic comparison for post-stroke AF detection rates, anticoagulation uptake and secondary stroke outcomes based on AF detection rates.
The Gap in the Clinical Practice of Cardiac Monitoring for Secondary Stroke Prevention
Adherence to the Guidelines and Recommendations for Prolonged Cardiac Monitoring
Prolonged cardiac monitoring following stroke increases the diagnostic yield of AF; this affects therapy prescribed for the secondary prevention of cerebral ischaemic events. Asian people are known to be at a higher risk of warfarin-related intracranial bleeding.51,52 Although the use of non-vitamin K oral anticoagulants (NOACs) favours Asian patients with AF in terms of risk reduction for stroke or systemic embolism and major bleeding events, results of large clinical trials have failed to demonstrate the superiority of NOACs over aspirin in preventing stroke recurrence after embolic stroke of undetermined source (ESUS).53−59 Oral anticoagulation therapy is recommended in patients with embolic stroke only if AF is known or detected based on current evidence.12,57 Therefore, it is important to identify AF after an ischaemic stroke before using NOACs.
Despite current guidelines recommending at least 24–72 hours of cardiac monitoring to detect AF after stroke, adherence to cardiac monitoring in real-world practice is relatively inconsistent.11,12 The Canadian Ontario Stroke Registry published in 2016, for example, found that only 30.6% of the patients who presented with first acute ischaemic stroke or TIA had 24-hour Holter monitoring in the first 30 days after the index event, and less than 1% of the patients underwent prolonged cardiac monitoring beyond 48 hours (in the first 90 days) after the onset of stroke.60 Another global survey that was published in the same year, the ESUS Global Registry (which characterised the features of patients with ESUS across five global regions, i.e. Europe, North America, Latin America, East Asia and the Pacific) found that 40% of patients with ESUS underwent only 24-hour Holter monitoring, 33% had inpatient cardiac telemetry, 19% had both 24-hour Holter monitoring and inpatient cardiac telemetry, and only 8% had extended (>24 hours) cardiac monitoring with Holter or non-telemetry cardiac rhythm monitoring procedures.61
Guidelines and Recommended Modalities for Screening for AF After Ischaemic Stroke
The current American Heart Association/American Stroke Association guidelines recommend CIED, Holter and cardiac telemetry monitoring strategies for prolonged cardiac monitoring in stroke patients, based on the data established from large trials.12,32,62–64
CIEDs represent the gold standard in cardiac monitoring in view of the long-term continuous monitoring associated with them. With the advent of long-term monitoring (up to 3 years), these insertable cardiac monitors detect paroxysmal AF that typically occurs in ischaemic stroke as infrequent brief episodes.
The new generation of CIEDs enable continuous remote patient monitoring with automated daily transmission and user-specified AF detection algorithms.65 In addition, the new monitors are smaller in size; this eases the implantation procedure, which is only minimally invasive. Nevertheless, their use is limited by several factors, including patient preference for non-invasive procedures and, most importantly, the high cost of the device and its implantation; the cardiac facilities and services required for implantation; and the often limited access in many developing Asian countries.
Even after excluding patients in whom AF is highly unlikely to have contributed to the incidence of stroke and those in whom a finding of AF would most probably not result in a change in management, the use of CIEDs for AF detection in stroke patients that is clearly clinically justified will still be a burden on healthcare systems in countries in which the population density is high and cardiac services are limited and in those with government-subsidised healthcare services.66
Holter monitoring is the most widely used diagnostic tool for non-invasive continuous cardiac monitoring in stroke patients. In one Asian study that included 1,315 patients with ischaemic stroke and TIA, Holter monitoring was initiated at a median of 4 days after the onset of stroke for a duration of 6 days and yielded an AF detection rate of 8.3%.34 Although there is extensive evidence supporting the use of Holter monitoring in the short–intermediate term, it is usually not readily available for immediate inpatient use in most hospital settings because of competing needs. Apart from the limited access to Holter monitors, the low usage of Holter monitoring in stroke patients after discharge is also due to poor patient adherence, in association with the rising cost and burden of outpatient Holter monitoring.67 Even in the context of clinical trials, adherence to intensive Holter monitoring decreases with extended duration. In the Find-AFRANDOMISED trial, which included 398 patients, two-thirds of the patients with stroke had AF that was detected in the first 10 days of monitoring, which commenced at a median of 4 days after the onset of stroke; the participation rate decreased continuously at the second and third 10-day monitoring periods after 3 and 6 months, respectively.68
In addition, Holter monitoring is often short-term, that is, for 24–48 hours, due to the potential inconvenience and discomfort caused by the wired external leads placed on the patients. Continuous cardiac ECG monitoring using inpatient cardiac telemetry equipped with an algorithm for detecting AF for at least 72 hours (as per the European Society of Cardiology recommendations) while patients are admitted for treatment addresses the limitations of outpatient Holter monitoring and limited availability of Holter monitors for immediate inpatient monitoring.11 However, as with Holter monitoring, cardiac telemetry has similar limitations with its wired external leads.
Alternative Modalities for Detecting AF after Ischaemic Stroke
The ideal mode (i.e. intermittent or continuous) and timing of monitoring for AF after an ischaemic stroke is not well understood.18 However, considering the practical limitations that restrict cardiac monitoring to a finite window, early monitoring is certainly advantageous for detecting AF based on the data published from both Asian and non-Asian cohorts (Supplementary Material Table 2). Early initiation of cardiac monitoring after an ischaemic stroke will potentially detect AF early and facilitate early commencement of oral anticoagulation therapy for secondary stroke prevention. Indeed, the superiority of oral anticoagulants to aspirin is evident in the prevention of vascular death, stroke, MI and systemic embolism if the treatment is initiated early (within 3 months) in patients with ischaemic stroke or TIA in whom AF is detected.69 In addition, early monitoring enables cardiac monitoring to be performed while patients are admitted to the ward for treatment. This enhances patient adherence and avoids the extra costs and burden associated with outpatient monitoring. The limitations of recommended cardiac monitoring modalities for the early initiation of extended cardiac monitoring, which include the cost of CIEDs; the requirement of extensive investigations to rule out all possible sources of stroke prior to implantation (which may delay the diagnosis of AF); the availability of Holter and/or cardiac telemetry for immediate inpatient monitoring; and the limitations associated with wired cardiac monitoring (which may result in poor patient adherence) can be addressed with the use of alternative cardiac monitoring modalities.
The patch ECG monitoring device consists of a cardiac monitor attached to a patch electrode and is applied to the left pectoral region of the chest.70–73 The patch device provides the same mechanism of continuous monitoring as the conventional Holter monitor, except that its added wireless and waterproof features make intermediate-term monitoring more tolerable (for up to 14 days).70–73 Some devices have incorporated an additional patient-activated event trigger function that enables clinicians to determine whether there is an association between patient symptoms and a cardiac event when reviewing the ECG strips.70–73 One study reported skin irritations associated with patch device monitoring, with some visible erythema and minimal oedema/popular rashes.71 Given that the market growth of patch monitoring technology is still in its infancy, the cost of monitoring using patch devices is relatively more expensive than that of the conventional Holter. We believe that patients will eventually benefit from economies of scale, when more evidence is established to support the use of this wireless Holter for intermediate-term monitoring; the indication for its use, including as an alternative method recommended in the guidelines, is prolonged cardiac monitoring.
The EMBRACE trial, which included 571 patients with cryptogenic stroke or TIA, applied the intermittent ECG monitoring strategy using an ambulatory event monitor, which was worn as a non-adhesive belt around the chest for 30 days, and an ECG recording event monitor.49 Mobile cardiac outpatient telemetry (MCOT), which was applied to 239 patients with cryptogenic stroke in the SMART registry, is worn around the neck with wires connecting the device to the chest electrodes. The MCOT records ECGs continuously and transmits data to a portable monitor to a built-in mobile phone.6 As with Holter, this device relies on a wired connection to the chest leads and thus has similar limitations to Holter in intensified outpatient cardiac monitoring. In fact, one clinical trial reported that patient adherence to prolonged cardiac monitoring using MCOT was less than 50%.67
The Evidence Gap in AF Detection for Secondary Stroke Prevention
Emerging Technologies for the Detection of AF After Ischaemic Stroke
Recently, mobile and digital ECG monitoring technologies have been successfully deployed in patients with AF. These technologies are inexpensive and require minimal training for use. Hence, they enable use at home; in community centres, clinics and pharmacies, and primary care settings; and as part of community outreach programs in the US, Europe and Asia.70−79 In Asia, mobile and digital ECG technologies have emerged as novel cardiac monitoring modalities for the detection of AF in community- and population-based studies to address the knowledge gap in the epidemiology of AF in this region.80−83
More evidence is required to establish the feasibility of these technologies in facilitating the early identification of AF after ischaemic stroke. Yan et al. and Koh et al. were among the first teams to evaluate the detection of AF using the hand-held smartphone ECG monitor after ischaemic stroke.36,38 Yan et al. reported that all of the ECG recordings were carried out by nurses as inpatient procedures in the ward during their observations of routine vital signs (every 2–4 hours).36 The study reported an AF diagnostic yield of 8.5% over a median duration of 4 days. Koh et al., in contrast, devised a different strategy: the smartphone ECG monitoring was performed as a patient-initiated procedure for patients who were admitted for ischaemic stroke or TIA in the preceding 12 months.38 Although the study reported an AF diagnostic yield of 9.5%, the authors acknowledged that the median adherence to outpatient mobile ECG monitoring was only 63.3%. They also reported other limitations, such as the compatibility of the smartphone with the hand-held mobile ECG device, and the patient’s socioeconomic conditions, which restrict the use of mobile ECG devices for outpatient monitoring. All of these limiting factors are common in many Asian populations (especially developing countries) and should be addressed when implementing this technology for the detection of AF in the stroke population.
The recent COVID-19 outbreak accelerated the uptake of mobile and digital technologies by the healthcare system with an increase in the usage of the remote patient monitoring strategy. In many Asian developing countries, the ability to reach out to low-resource and geographically isolated populations is the major strength of mobile and digital health technologies; however, this is only made possible with the accompanying acceleration of digital connectivity and the development of communication strategies. Given the high burden of AF and stroke in Asia, the characteristic incorporation of automated artificial intelligence algorithms into mobile and digital technologies for AF identification appropriately addresses the issues associated with the healthcare system in many developing Asian countries, where limited resources (expertise and facilities) are already overstretched.13–15,17 First, the centralisation of expertise is possible with the advent of digital health in remote patient monitoring. Second, while reaching out to a wider population, it also helps to reduce inefficient processes and enable healthcare providers to attend to patients more effectively. Koh et al. reported that of the 108 patients who underwent smartphone ECG monitoring, 48.6% received at least one notification for possible AF that required further verification; of these, true-positive AF was identified in approximately one-quarter of the ECGs that were classified as possible AF.38 Further studies are warranted to evaluate the ideal deployment of mobile and digital technologies to support intermediate- and long-term monitoring for AF detection.
Cost-effectiveness of Emerging Technologies for Detection of AF After Stroke
Cost-effectiveness analyses of these emerging, non-invasive monitoring tools for the identification of AF after stroke are also lacking, particularly in the context of the Asian population. Yong et al. assessed the cost-effectiveness of an intermittent ECG monitoring strategy using an ambulatory event monitor in the Canadian cohort from the EMBRACE trial and reported that 30-day monitoring (at a cost of US$447) was cost-effective (US$2,000 per quality-adjusted life year [QALY] gained). Cost-effectiveness was sensitive to stroke recurrence risk and anticoagulant effectiveness.84 For the smartphone ECG monitoring, an economic analysis of the Australian cohort from the Yan et al. study reported that the mobile ECG recording (during hospital stay) is a highly cost-effective strategy (AU$3,013 per QALY gained) for the secondary prevention of stroke. Mobile ECG monitoring during index hospitalisation was associated with marginally increased costs (AU$31,196) and improved health outcomes (6.70 QALYs) compared with 24-hour Holter surveillance (AU$31,095 and 6.66 QALYs) over a 20-year time horizon and contributed to lower recurrence of stroke and stroke-related deaths (140 recurrent strokes and 20 deaths avoided per 10,000 patients).85 Cost-effectiveness analyses examining the economic credentials of these emerging cardiac monitoring technologies for post-stroke AF screening are warranted, especially for Asian patients, in future analyses.
Threshold for the Diagnosis of AF and Secondary Stroke Prevention
Most of the Asian studies in this review adopted a duration of ≥30 seconds as the definition of AF.33,34,36,38,40,43 The 30-seconds duration was first defined in the HRS/EHRA/ECAS Expert Consensus Statement on Catheter and Surgical Ablation of Atrial Fibrillation: Recommendations for Personnel, Policy, Procedures and Follow-up published in 2007 and has been retained in the current 2017 guidelines.86,87 However, in the context of stroke, the threshold for AF that warrants switching patients from antiplatelets to anticoagulation therapy for secondary stroke prevention still remains unclear and the current practices vary between clinicians. A higher burden of AF has been shown to increase the risk of stroke; however, the threshold of AF that is associated with increased stroke risk is poorly understood.88−90 The majority of the studies reported AF detection rates but not anticoagulation decisions based on AF detection or stroke recurrence rates. Seow et al. reported an anticoagulation uptake of 90.9% in patients in whom >2 minutes of AF was detected on a CIED; Huang et al. found that for patients with ischaemic stroke in whom AF was detected (AF duration undefined), 37.1% of patients in the serial ECG group and 39.3% in the 24-hour Holter group were on oral anticoagulation at discharge; and 51.4% in the serial ECG group and 64.3% in the 24-hour Holter group were on oral anticoagulation at 3 months after their index stroke events; while Yan et al. found that only 44% of the patients in whom AF lasting >30 seconds was detected were started on anticoagulation therapy.35,36,42
In the western population, although some guidelines propose 2–6 minutes and others >24 hours as the AF threshold at which oral anticoagulation therapy should be commenced, these recommendations were based mainly on investigations that linked AF to the occurrence of the first stroke.65,88,90−94 The threshold of AF remains arbitrary, and some believe that a shorter duration of AF (<30 seconds), particularly if detected after an index stroke, could be clinically relevant and should be considered as occult AF. However, this remains a hypothesis while there is no established evidence to correlate the increased risk of the recurrence of stroke with paroxysmal AF lasting <30 seconds or the reduction in the recurrence rate of stroke in patients (who are anticoagulated) with stroke and paroxysmal AF lasting <30 seconds. Whether a single episode of AF lasting 30 seconds–2 minutes or 12 episodes lasting 10 seconds, for instance, is clinically relevant based on the risk of stroke recurrence is unknown.
The current CHAsDS2-VASC risk assessment criteria categorised patients with stroke and AF into the high-risk category (2 points for stroke). The questions of whether merely detecting AF (of any duration) or a certain threshold of AF warrants switching patients with ischaemic stroke from antiplatelets to anticoagulation therapy, and whether the effect of threshold on the diagnosis of AF differs by ethnicity (i.e. Asian versus Western), requires further investigation with large outcome-driven trials to evaluate larger cohorts of patients with stroke and AF.
Conclusion
Data evaluating the detection of AF in patients with ischaemic stroke in Asia are limited. From data published in the Asian population, we propose early initiation of cardiac monitoring in patients admitted with acute ischaemic stroke or TIA. More evidence supporting the indication and use of novel technologies for prolonged cardiac monitoring to detect AF is required to address the limitations of the guidelines and recommended modalities for early and prolonged cardiac monitoring. Early initiation of prolonged cardiac monitoring in patients with ischaemic stroke or TIA will lead to meaningful, clinically relevant outcomes only when it is coupled with anticoagulant uptake. Therefore, the threshold of AF that warrants anticoagulation therapy needs to be better defined in future large outcome trials evaluating patients with stroke and AF.
Clinical Perspective
- Stroke due to AF is common and frequently devastating. However, up to 40% of AF cases in stroke patients remain paroxysmal and clinically silent, making diagnosis challenging.
- Prolonged cardiac monitoring increases the diagnostic yield of AF. In Asia, where the population density is high and there is competition for access to limited resources, monitoring strategies should be selected with considerations for local and regional challenges.
- Innovative technologies such as patch monitors and mobile and digital devices address the limitations of recommended monitoring technologies for Asian patients with stroke, which are often associated with cost, accessibility and patient preference. Further studies are warranted to generate evidence-based recommendations that support the deployment of these technologies for patients with stroke as alternative monitoring modalities to detect AF.
- Early initiation of cardiac monitoring after an acute ischaemic stroke or transient ischaemic attack should be considered in view of practical limitations that restrict monitoring to a finite window in many Asian settings. Early monitoring should also be indicated for the early detection of AF; this helps to start oral anticoagulation therapy to prevent a second and more debilitating stroke.
- Future investigations should focus on defining thresholds for the diagnosis of AF based on decisions for anticoagulation therapy, reduction of stroke recurrence, and the duration of monitoring to reduce stroke recurrence while increasing the AF detection rate.