“If you know the enemy and know yourself, your victory will not stand in doubt.”1
The Art of War, an ancient military treatise composed around the 5th century BCE, stressed the importance of knowing the enemy to adopt different strategies when fighting the enemy, to attack, to surround, to avoid, to battle, to flee.2
Clot is often the enemy during primary percutaneous coronary intervention (PCI). Interventionists can take inspiration from the ancient wisdom to fight the enemy. The first step is to assess the potential impact of the thrombus on PCI and clinical outcome. Steps to follow include consideration of the various strategies for managing a thrombus, then to adopt the appropriate strategy and algorithm based on the perceived significance of the thrombus and its response to an individual strategy.
Impact of Thrombus on Percutaneous Coronary Intervention and Clinical Outcome
Intracoronary thrombus can lead to occlusion of an epicardial vessel or its branches during PCI and can cause distal embolisation resulting in distal vessel occlusion and no-reflow. The presence of coronary thrombus during PCI has been shown to be associated with adverse procedural and clinical outcomes, including no-reflow, MI, emergency bypass surgery and in-hospital mortality.3–5
Predictors of Worse Outcome: Thrombus Burden
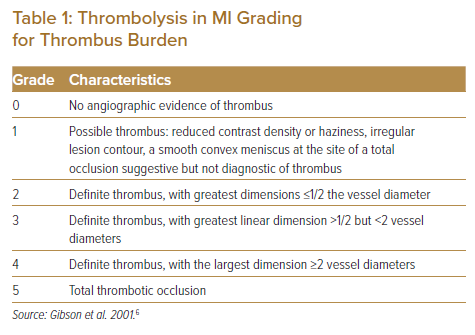
Thrombus burden is a predictor of a worse outcome. The most widely used tool to assess thrombus burden is the Thrombolysis in MI (TIMI) thrombus grading, which is largely based on the dimension of thrombus relative to the vessel size (Table 1).6 High TIMI thrombus grade has been shown to be a predictor of distal embolisation in primary angioplasty.7 As a practical approach, a simplified bi-level categorisation has been proposed, whereby TIMI grades 1–3 are classified as low grade while TIMI grades 4–5 are classified as high grade.8 Notably, total thrombotic occlusion is given the highest grade of 5, but the actual thrombus load in a totally occluded lesion is uncertain. In fact, after the exclusion of patients with patent infarct-related artery, TIMI grade 5 became not to be regarded as an independent predictor of distal embolisation.7 Thus, when total thrombotic occlusion is encountered, it is recommended to first establish flow by guidewire crossing or by passage of a non-inflated balloon. Thereafter, the thrombus grading can be re-stratified to TIMI grade 1–4, with grade 1–3 indicating a small thrombus burden and grade 4 representing a large thrombus burden. By this approach, a large thrombus burden of grade 4 has been reported to be a predictor of stent thrombosis and major adverse cardiac events (MACE) at 2 years.9
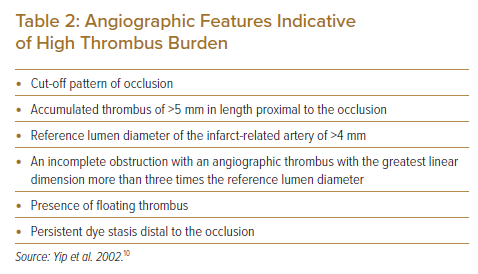
Thrombus burden can also be assessed by angiographic morphologic features as proposed by Yip et al (Table 2).10 These features were shown to be independent predictors of slow-flow and no-reflow after primary PCI, which in turn was associated with higher 30-day mortality.10
It should be noted that coronary arteries of different sizes with identical TIMI thrombus grades may well have considerably different absolute thrombus burdens. Thus, both TIMI thrombus grade and angiographic features should be considered when assessing thrombus burden, to determine the strategy for intervention.
Beyond Thrombus Burden: The Impact of Thrombus Composition
The prognosis when dealing with intracoronary thrombus is determined not only by thrombus burden but also by thrombus composition. Red thrombus, compared with white thrombus, tends to be more resistant to intervention and is associated with worse clinical outcomes including 30-day MACE and mortality.11 Different characteristics of fibrin network also affect the behaviour of thrombus to intervention.12 However, there are limited clinical data on adopting specific interventional strategies based on assessment of thrombus composition. Interventionists should anticipate variable responses of thrombus to intervention and be ready to escalate treatment strategy as needed.
Strategies to ‘Fight’ the Thrombus
To Attack: Thrombectomy
Manual Aspiration Thrombectomy: Does a Class III Recommendation Mean an Obsolete Weapon?
The TAPAS trial was an early, relatively large, single-centre trial randomising 1,071 ST-elevation MI (STEMI) patients to aspiration thrombectomy versus conventional PCI. Aspiration thrombectomy improved myocardial reperfusion, evident by better myocardial blush grade (blush grade 0–1, 17.1% versus 26.3%; p<0.001) and more complete resolution of ST-segment elevation (56.6% versus 44.2%; p<0.001).13 Moreover, there was a significant reduction in cardiac (3.6% versus 6.7%; p=0.02) and all-cause mortality (4.7% versus 7.6%; p=0.042) at 1 year.14 However, this positive result was not reproduced in two subsequent larger multicentre trials – the TASTE trial and the TOTAL trial – that randomised 7,244 and 10,732 patients, respectively.15,16 The TASTE trial did not show benefit of aspiration thrombectomy in all-cause mortality at 30 days (2.8% versus 3.0%; p=0.63) or 1 year (5.3% versus 5.6%; p=0.57). There was also no difference in the incidence of hospitalisation for recurrent MI and stent thrombosis.15,17 The TOTAL trial also did not demonstrate benefit in primary composite outcome of death from cardiovascular causes, recurrent MI, cardiogenic shock, or New York Heart Association class IV heart failure within 180 days (6.9% versus 7.0%; p=0.86). More remarkably, the TOTAL trial indicated a safety concern, with an increased incidence of stroke within 30 days in the aspiration thrombectomy group (0.7% versus 0.3%; p=0.02).16 With the evolution in data, major guidelines downgraded the routine use of aspiration thrombectomy in primary PCI to a class III recommendation.18,19
Of note, the clinical evidence only indicates against routine use of aspiration thrombectomy; it should not deter the use of aspiration thrombectomy altogether. Instead, it calls for mindful selected use, notably for cases with high thrombus burden. Moreover, it demands technical consideration to minimise the potential risk of stroke related to the procedure.
The major trials described were all-comer trials and did not specify thrombus burden as eligibility criteria, bringing uncertainty about potential benefits of aspiration thrombectomy in patients with high thrombus burden.13,15,16 Subgroup analysis for patients with high thrombus burden in the TASTE trial (included TIMI thrombus grade 4–5) and TOTAL trial (included TIMI thrombus grade 3–5) revealed similar findings of lack of benefit.15,17,20 However, uncertainty still exists because of study limitations. In TASTE, the subgroup of high thrombus burden constituted only 31% of the whole study population, which reduced the power of the study.15,17 In TOTAL, the subgroup constituted a much higher 84% of the initial study population. However, assessment of thrombus grading in TOTAL was performed before wire crossing. A high 72% of this subgroup belonged to TIMI thrombus grade 5, which precluded accurate assessment of actual underlying thrombus burden before wire or balloon crossing.20 Putting together the current evidence, it is not unreasonable to perform aspiration thrombectomy for patients with high thrombus burden, but the thrombus burden needs to be confirmed by wire or balloon crossing in case of total thrombotic occlusion on initial coronary angiography.
The TOTAL trial and meta-analyses alerted us to the potential risk of stroke in association with the use of aspiration thrombectomy, although the absolute risk was small.16,21–23 The increase in incidence of stroke within 48 hours raised concerns that it might be procedure-related.24 There were some uncertainties though. There was also a trend of increase in stroke occurring late after 7 days, in particular between 90 and 180 days. In addition, there was a numerical increase in haemorrhagic stroke. Both were not explainable as being related to the procedure.24 Moreover, an increase in risk of stroke was not demonstrated in TASTE and other meta-analyses.15,25,26 Despite these uncertainties, when performing aspiration thrombectomy it is prudent to take precautions to minimise the risk of stroke as a result of aspirated thrombus being dislodged into the systemic circulation or inadvertent injection of air that is entrapped in the guiding catheter during manipulation of the aspiration catheter. During withdrawal of the aspiration catheter, the guiding catheter should be kept engaged and excessive manipulation should be avoided. Constant negative pressure should be maintained on the aspiration catheter during retrieval until it is removed from the guiding catheter. The guiding catheter should then be allowed to bleed back to clear any remaining thrombus or entrapped air before taking further angiographic images.
Mechanical Thrombectomy: Where Does It Stand in the Battle?
Mechanical thrombectomy is less extensively studied than manual aspiration thrombectomy. The AIMI trial randomised 480 patients undergoing primary PCI to routine adjunctive use of AngioJet rheolytic thrombectomy (Boston Scientific) versus PCI alone. The rheolytic thrombectomy group showed worse outcomes in final infarct size, and achievement of TIMI flow grade 3 and 30-day MACE.27 The JETSTENT trial studied 501 patients with high TIMI thrombus grade 3–5. The rheolytic thrombectomy group did not show benefit in the primary endpoint of infarct size and surrogate endpoints of angiographic parameters of perfusion, while a benefit was shown in MACE at 6 months and 1 year.28 With the available data, major guidelines do not recommend routine use of rheolytic thrombectomy.18,19
The Indigo Aspiration System (Penumbra) is another option for mechanical thrombectomy that has been used in coronary thrombosis in recent years, although clinical data are still limited. The ROPUST study is a recent single-centre retrospective review of the use of Penumbra thrombectomy in 123 patients. TIMI 3 flow was achieved in 90.2%, compared to 87% of TIMI 0 or 1 flow at baseline.29 The CHEETAH study is a single-arm, prospective multicentre study of 400 patients with TIMI thrombus grade 4 or 5. Preliminary data showed that TIMI thrombus grade was 92.4% at grade 4–5 at baseline; this was improved to 72.8% at lower than grade 4 after thrombectomy, and 99.5% at grade 0 at the end of the procedure.30 In both series, no device-related serious adverse events were reported.29,30
Another novel approach is mechanical thrombectomy using the Solitaire device (Medtronic), which has been shown to be safe and effective in the treatment of acute ischaemic stroke. Initial experience in a small cohort of patients showed that the device was applicable to intracoronary thrombus and was highly efficacious in reducing thrombus burden and improving TIMI flow in patients with high thrombus burden despite other conventional therapies.31 The effectiveness of the Solitaire device for refractory thrombus in acute coronary syndrome is being investigated in a larger multicentre open-label prospective feasibility trial.32
Currently, clinical outcome data are insufficient to support the routine use of mechanical thrombectomy. However, AngioJet rheolytic thrombectomy, Penumbra thrombectomy (Indigo Aspiration System) and the Solitaire device are efficacious in thrombus removal. As an individualised clinical decision, it is sensible to adopt mechanical thrombectomy for patients with high thrombus burden, either when thrombo-aspiration is not effective or as first-line when the thrombus burden is perceived to be very high.
To Surround: Pharmacological Therapy
Other than ensuring adequate anticoagulation throughout the PCI procedure, various pharmacological therapies play a key role in dealing with coronary thrombus.
Oral Anti-platelet Pre-treatment
For STEMI patients undergoing primary PCI, dual antiplatelet therapy with aspirin plus a potent P2Y12 inhibitor (ticagrelor or prasugrel) is recommended as the standard pre-treatment to reduce thrombotic events.18,33 It has been shown to improve outcomes compared with aspirin plus clopidogrel.34,35
Cangrelor
Cangrelor is an IV P2Y12 receptor antagonist characterised by rapid onset of action, potent P2Y12 inhibitory effects and fast off-set of effects. In the CHAMPION Phoenix trial, cangrelor was compared with clopidogrel across the full spectrum of coronary artery disease, with 18% being STEMI. Cangrelor significantly reduced the primary end point of death, MI, ischaemia-driven revascularisation, or stent thrombosis at 48 hours.36 However, the clinical benefit of cangrelor over the more potent P2Y12 inhibitor is not well studied. A small open-label randomised trial in primary PCI setting showed that cangrelor, when compared with ticagrelor, achieved significantly greater P2Y12 inhibition at first balloon inflation time, but there was no difference in final infarct size, angiographic and electrocardiographic measures of reperfusion.37
In practice, cangrelor may be used if a patient cannot take or cannot tolerate oral antiplatelet pre-treatment, such as when the patient is intubated or experiencing vomiting.
Glycoprotein IIb/IIIa Inhibitors
Various studies have indicated the benefits of glycoprotein IIb/IIIa inhibitors (GPI) in STEMI, including survival benefit.38 However, the clinical benefits were demonstrated in an era before the use of routine dual antiplatelet therapy, potent P2Y12 inhibitors, thrombectomy and contemporary stents. Pre-hospital routine upstream use of GPI has also been studied, but did not indicate a benefit and thus is not recommended.18
In brief, routine use of GPI in primary PCI is not recommended. Nonetheless, it is reasonable to use GPI when high thrombus burden is present, especially if thrombectomy has been attempted but there is persistent significant residual thrombus.
GPI given through the intracoronary route rather than IV route has the theoretical advantage of providing a higher local concentration, augmenting thrombus resolution. Some small studies and meta-analyses showed benefit, but this was not demonstrated in a large randomised trial. 39,40 While there were no consistent findings to indicate a benefit, there was also no indication of harm. Thus, the intracoronary route of GPI may be considered according to the interventionist’s preference and discretion.
Intracoronary Thrombolytics
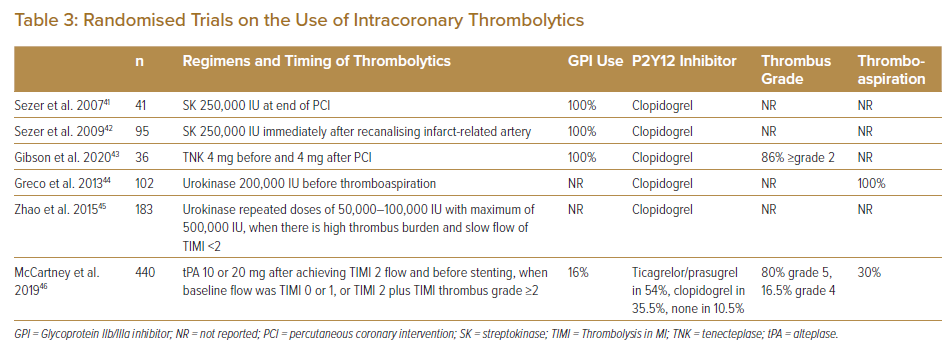
Following early anecdotal reports and small series in the use of intracoronary thrombolytics, randomised controlled trials were carried out assessing various endpoints including epicardial flow, microvascular perfusion, infarct size, left ventricular function and MACE.41–46 These were relatively small studies with study populations ranging from 36 to 440. Meta-analysis of these trials indicated that intracoronary thrombolysis was associated with a higher likelihood of ST-segment resolution and a strong trend toward reduction in in-hospital MACE, with no sign of increased bleeding.47 Notably, individual studies varied widely in design (Table 3). The intracoronary thrombolytics might be given after recanalising the infarct-related artery, at end of PCI, both before and at end of PCI, before thrombo-aspiration, when slow-reflow develops in association with high thrombus burden or when angiography showed TIMI 0/1 flow or TIMI 2 flow in associated with thrombus grade ≥2.41–46 The regimens of thrombolytic also varied, including streptokinase 250,000 IU, urokinase up to 500,000 IU, alteplase up to 20 mg or tenecteplase 8 mg in two doses; these were equivalent to 10–25% of systemic dosages.41–46 The concurrent P2Y12 inhibitors used were all clopidogrel in five studies.41–45 In one study, which was the largest and comprised about half of the patients in the meta-analysis, ticagrelor or prasugrel was used in 54% of patients.46 GPIs were used by protocol in all patients in three studies, in 16% in one study and not reported in the other two studies. 41–46
With the small number of patients studied and the heterogenicity in study designs, the precise beneficial role of intracoronary thrombolytics still needs to be defined. Nevertheless, the findings to date are encouraging, and it is reassuring that there was no signal of significant bleeding risk, although potent P2Y12 inhibitors were not used in the majority of patients in those studies. Taken together, low-dose intracoronary thrombolytics may be considered for large thrombus burden, especially if not responsive to other more conventional treatments.
To Avoid: Stenting Strategies to Minimise Manipulation of Thrombus
Direct Stenting
During primary PCI with thrombotic lesion, distal embolisation resulting in occlusion of distal epicardial arteries or microvascular obstruction is common and is associated with worse clinical outcomes. Direct stenting has been proposed to minimise manipulation of thrombus and to fix the thrombus behind the stent to reduce the occurrence of distal embolisation. However, there are also theoretical disadvantages of direct stenting, such as underestimation of true vessel size, difficulty in passage of stent through a tight or calcific lesion, stent under-expansion and late malapposition; all these may increase the risk of stent restenosis or stent thrombosis. A meta-analysis of five small randomised controlled trials, with a total of 754 patients, indicated that direct stenting improved ST-segment resolution and was associated with a reduced risk of in-hospital cardiovascular death. There was no difference in target lesion revascularisation.48 More recently, the effect of direct stenting was studied in a large propensity-matched cohort of 10,944 patients originally enrolled in the three major trials of aspiration thrombectomy. Patients randomised to thrombus aspiration showed a significantly higher rate of direct stenting as compared with PCI only (41% versus 22%). Direct stenting did not indicate a benefit in measures of myocardial perfusion or clinical outcomes at 30 days and 1 year. However, there was no evidence of harm regarding incidence of stent thrombosis or target vessel revascularisation.49
Without clear demonstrable benefit, the decision for direct stenting should primarily be based on lesion anatomy. Although aspiration thrombectomy can facilitate direct stenting, it should not be performed solely for this purpose.
Deferred Stenting
Deferred stenting is another proposed strategy to reduce the risk of distal embolisation of thrombus and no-reflow, with the aim that the deferred strategy allows time for the thrombus to partially resolve and for microvascular dysfunction on presentation to recover.
The DEFER STEMI study randomised 101 STEMI patients to deferred stenting versus conventional PCI when TIMI 3 flow was achieved but there were clinical or angiographic risk factors for no-reflow.50 Deferred stenting, at a median of 9 hours, demonstrated benefits in significant reduction in no-/slow-reflow and intraprocedural thrombotic events, and greater myocardial salvage index at 6 months. However, no significant benefit was shown in subsequently published studies. DANAMI 3-DEFER was a large multicentre study with 1,215 patients. Unlike the DEFER STEMI study, it randomised patients on a routine basis, not mandating the presence of high-risk features. The median time to deferred stenting was 3 days. The study showed no difference in clinical outcomes of all-cause mortality, hospital admission for heart failure, recurrent MI, or unplanned revascularisation of the infarct-related artery.51 Notably, both the DEFER STEMI and DANAMI 3-DEFER studies reported a remarkable re-occlusion rate of 4% and 2%, respectively, before the planned second PCI.50,51 The MIMI and INNOVATION studies were two other smaller trials that randomised 140 patients and 114 patients, respectively, on routine basis.52,53 The two studies revealed no significant benefit in microvascular obstruction or infarct size, while INNOVATION study showed benefit in both measures in the subgroup of anterior MI. 52,53
Meta-analyses indicated that deferred stenting did not show benefits in hard clinical endpoints such as MI, cardiovascular death and all-cause death. Some showed improved outcomes in no-/slow- reflow or surrogate angiographic endpoints like TIMI flow and myocardial blush grading.54–57
From the current evidence, deferred stenting as a routine strategy is not appropriate. However, it may be considered in the presence of high residual thrombus burden after initial treatment with thrombectomy and pharmacological therapy, and when the patient is considered at high risk for no-/slow-reflow after stenting. The ideal deferral time is not well defined by clinical data. Practically, a deferral timeframe of approximately 5 days is reasonable, balancing the time required for more optimal thrombus resolution and avoiding excessive delays in completing PCI. Noting the risk of early re-occlusion, it is prudent to adopt deferred stenting only after at least TIMI 2 flow – preferably TIMI 3 flow – is achieved and there is stable flow after initial treatment. Moreover, it is recommended to put the patient on GPI and heparin unless contraindicated, which is also the strategy commonly used in studies.
To Battle: Stenting Residual Sizable Thrombus
In the presence of sizable residual thrombus after initial treatment, there are situations where stenting can be performed under special consideration and strategies, as an alternative to consideration of deferred stenting.
Distal Protection Devices
The most widely used distal protection device is the distal filter device. Two early randomised trials studied the use of distal filter devices: one in either STEMI or non-STEMI (68.5% being STEMI) with angiographic evidence of thrombus and one in all-comers of STEMI (72% having visible thrombus). 58,59 Both did not show improvement in perfusion, infarct size or clinical outcome. These findings do not support the routine use of distal protection devices in primary PCI. For this reason, together with the availability and efficacy of other strategies in dealing with thrombus, the role of distal protection devices has become limited.
However, distal protection devices may still be useful for highly selected cases where significant thrombus burden persists after thrombectomy and pharmacological means. When the thrombi are mainly localised to the proximal to mid segment while there is a more distal segment that is sizable and free of thrombus and disease, the use of a distal protection device may be considered, also taking into account whether the coronary anatomy is suitable for placing the device.
Stenting Across Local Obstructive Thrombus
After initial therapy for large thrombus, there are occasions when the flow remains TIMI 0 or 1 because of local obstruction by residual thrombi in the proximal or mid-segment, instead of related to no-reflow or distal vessel thromboembolism. Confirmation of the latter can be achieved by angiography using contrast injection through a dual lumen catheter placed in the distal vessel, which is being pulled back while contrast injection is maintained. In this scenario, stenting across the local thrombus to ‘splint’ the thrombus can be helpful to maintain lumen patency and restore flow. A slightly undersized stent may be considered in this setting to minimise the risk of distal embolisation and no reflow after stenting, yet this is more a personal practice rather than based on clinical evidence.
To Flee: When Flow Cannot Be Regained
There are cases that fail to achieve more than TIMI 1 flow despite adopting various strategies for managing thrombus and no-reflow, with reported incidences in the range of 1–2.5%.51,60,61 This may be because of extensive thrombosis in an epicardial artery, distal embolism leading to no-reflow or other causes of microvascular dysfunction related to acute ischaemia. PCI needs to be adjourned. The patient should be maintained on optimal pharmacological therapy, notably GPI with or without a course of anticoagulant. Pharmacological and mechanical circulatory support should be provided as indicated by the patient’s haemodynamic parameters. This allows time for the thrombus to resolve, microvascular function to recover and – hopefully – for some myocardium to be salvaged.
Conclusion
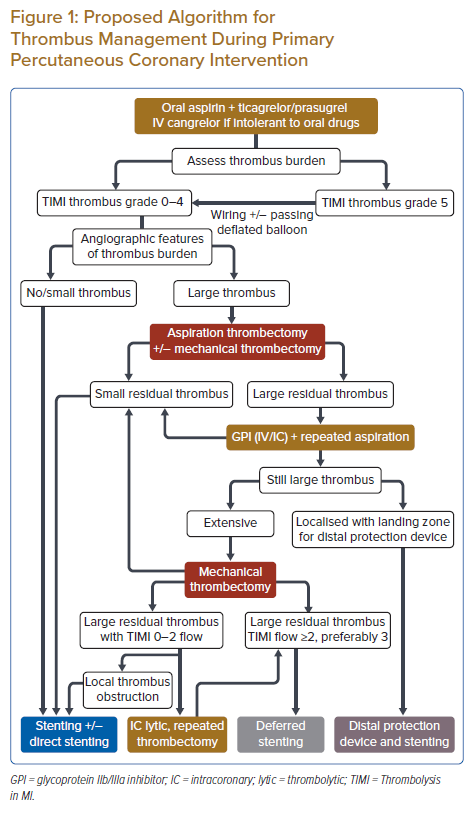
Clot is often the enemy during primary PCI. To deal with thrombus, interventionists can take inspiration from the ancient wisdom of fighting a war. The first step is to look at the strength of the enemy – in this context to assess the thrombus burden by both TIMI thrombus grade and angiographic features. This is to be followed by choosing the appropriate strategy: to attack – aspiration and mechanical thrombectomy for large thrombus burden; to surround – pharmacological therapy, including dual antiplatelet therapy with potent P2Y12 inhibitor as standard therapy, IV cangrelor if oral antiplatelet agents are not tolerated, IV or intracoronary GPI for large thrombus and intracoronary thrombolytic therapy for refractory large residual thrombus; to avoid – a stenting strategy including direct stenting if anatomy is suitable, and deferred stenting when extensive thrombus persists while TIMI flow ≥2, preferably 3, can be achieved; to battle – stenting under a distal protection device if anatomy is feasible, or stenting across local obstructive thrombus; or to flee – moving from PCI to an optimal antithrombotic therapy and circulatory support when flow fails to be regained. A proposed algorithm is shown in Figure 1. Of note, most of these strategies have not been demonstrated to be beneficial on routine use. The key remains to adopt different strategies selectively and appropriately in different scenarios.
Clinical Perspective
- Thrombus is often the ‘enemy’ in primary percutaneous coronary intervention.
- There are various ways to fight the enemy: thrombectomy, pharmacological agents including oral and IV antiplatelet agents and intracoronary thrombolytics, and various stenting strategies, such as deferred stenting.
- Routine use of most of these strategies is not supported by clinical data.
- The key is to adopt different strategies selectively and appropriately.