With continued advances in valve design and accumulated operator experience there has been improved clinical outcomes in the field of transcatheter aortic valve replacement (TAVR), with a resulting extension of TAVR as a treatment option beyond the high-risk patient to the low-risk subset of patients.1
There exist distinct technical and clinical differences between the various types of TAVR valves, which are inherent to the differences in valve design and deployment. Balloon-expandable valves (BEVs) typically adopt an intra-annular position, have a shorter frame length, and exert a higher radial force during deployment, whereas self-expanding valves (SEVs) are repositionable, may adopt an intra-annular or supra-annular position, have a longer frame length, and exert a smaller but sustained radial force beyond their nominal diameter.2 These factors may translate into potential differences in valve haemodynamics, paravalvular leak (PVL) and conduction disturbances, which may have important implications for valve durability, functional capacity, rehospitalisations and quality of life.3–5 Data on mortality between valve types are also limited and conflicting.6,7 With the inclusion of the low-risk subset of patients who may be younger with more remaining years of life, there exists a greater need to understand the possible differences in outcomes between BEV and SEV to guide valve selection for the individual patient.8 To date, several meta-analyses have compared outcomes of the different transcatheter heart valve types, but the vast majority are lacking long-term data beyond 1 year.
In this updated meta-analysis, we aim to evaluate differences in short- and longer-term clinical outcomes between BEV and SEV.
Methods
Literature Search
This meta-analysis was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines.9 We performed an electronic search of the MEDLINE database up to and including April 2020 using the keywords ‘transcatheter aortic valve replacement’, ‘transcatheter aortic valve implantation’, ‘aortic stenosis’, ‘balloon expandable’, ‘self-expanding’ and ‘self-expandable’. Permutations of the search terms were explored until no further new or relevant articles were generated. The reference lists of all review articles were searched for potentially relevant studies. Only studies published in English were included. The process of searching and reviewing was performed by two independent evaluators (EG, YSK) and discrepancies were discussed to achieve a consensus.
Selection Criteria
Studies were included in the meta-analysis if they fulfilled the following criteria: reported on the outcomes of BEV versus SEV; study design structured as a prospective or retrospective observation study or randomised controlled trial; minimum study duration of 30 days; and study population ≥50 subjects. Studies were excluded based on at least one of the following: study population <50 subjects; a focus on non-native aortic valve stenosis (e.g. valve-in-valve TAVR); and a focus on transapical TAVR.
Definitions
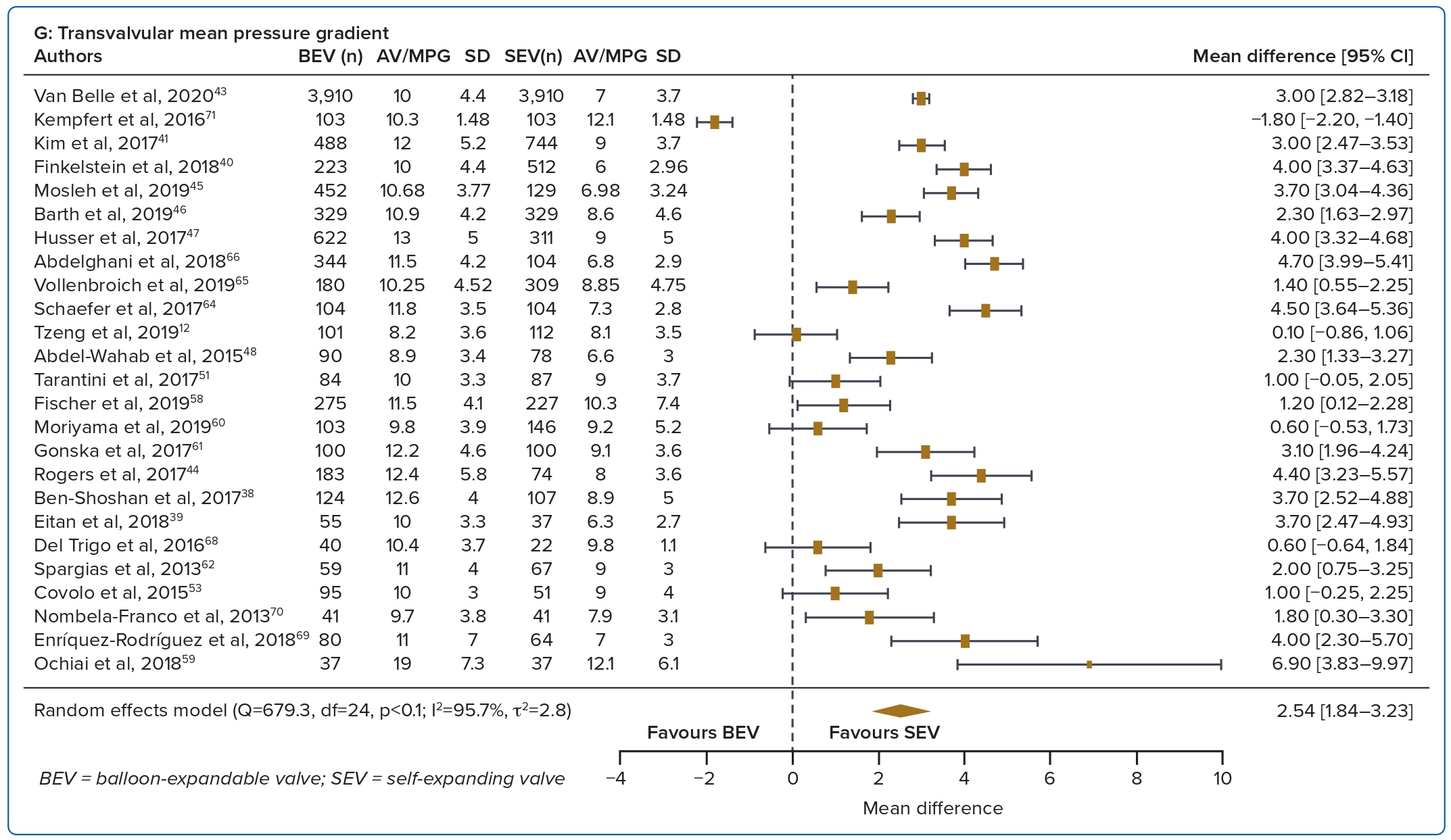
Study outcomes were divided based on time period into short term (in-hospital or 30 days), intermediate term (1 year) and long term (3 years). Data at other intervals (e.g. 2 years and 5 years) were not included due to the small number of studies. The primary outcome was all-cause mortality. Secondary endpoints included stroke or transient ischaemic attack (TIA), life-threatening or major bleeding, at least moderate PVL, permanent pacemaker (PPM) implantation, aortic valve area (AVA) and aortic valve mean pressure gradient (AV MPG).
Data Extraction
Studies were first screened at the title and abstract level. Studies matching the inclusion criteria were analysed at the full-text level. Two authors (EG, YSK) extracted the data independently, including type of study, number of cases, baseline characteristics (including age, gender, Society of Thoracic Surgeons (STS) score, EuroSCORE II, and type of access), duration of follow-up, and definition of outcomes. The BEVs consisted of the SAPIEN/SAPIEN 3/SAPIEN XT series. The SEVs consisted of the CoreValve/Evolut R/Evolut PRO series, the ACURATE/ACURATE Neo series and Portico.
Statistical Analysis
Meta-analysis using a random-effects model was performed to determine the pooled effect estimates. The estimators of difference were expressed as weighted mean difference with 95% CIs for continuous outcomes and ORs for dichotomous outcomes (i.e. stroke or TIA, mortality, and bleeding). When the mean was not reported, we estimated the mean using the median, and the standard deviation using the interquartile range with an assumption of normal distribution. Assessment of bias was completed on all included studies using funnel plots to visually display evidence of small-study effects. Heterogeneity was assessed using a homogeneity test based on the χ2 test. The I2 statistic was used to assess the impact of heterogeneity on the results.10 I2 values <25% represented low, values of 25–50% represented medium, and values >50% represented high heterogeneity. Two-tailed p<0.05 was considered statistically significant. All statistical analysis were performed using R version 4.1.1 (R Foundation) using the metafor package.11
Results
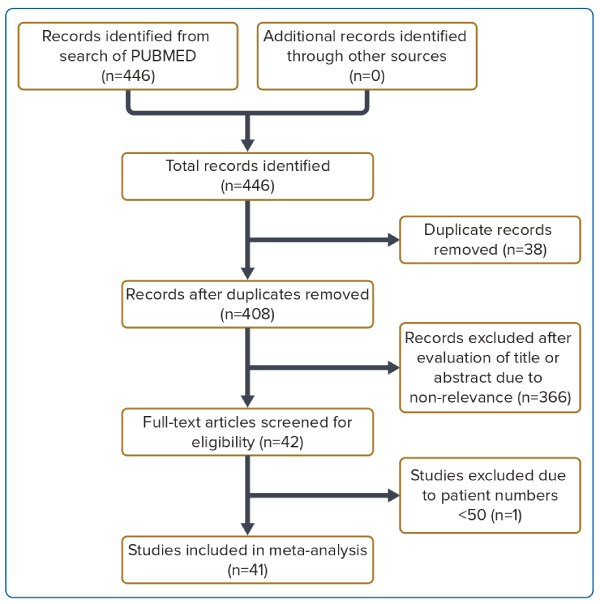
We identified a total of 41 studies comprising a pooled population of 46,000 patients of whom 23,892 (51.9%) had BEV implantation and 22,055 (47.9%) had SEV implantation (Figure 1). Valve type was unknown for 53 patients (0.1%). All BEVs were from the SAPIEN/SAPIEN 3/SAPIEN XT series. Of the SEVs, 19,920 (90.3%) were from the CoreValve/Evolut R/Evolut PRO series, 1,882 (8.5%) were from the ACURATE/ACURATE neo series, and 253 (1.1%) were Portico. Patients with Lotus valves were excluded from our analysis (n=18) because this valve has been withdrawn by the manufacturer.12 The baseline characteristics, including mean age, proportion of male patients, and STS score are listed in Supplementary Table 1. There were no significant differences in age or STS score between the BEV and SEV studies.
Short-term Outcomes (30 days)
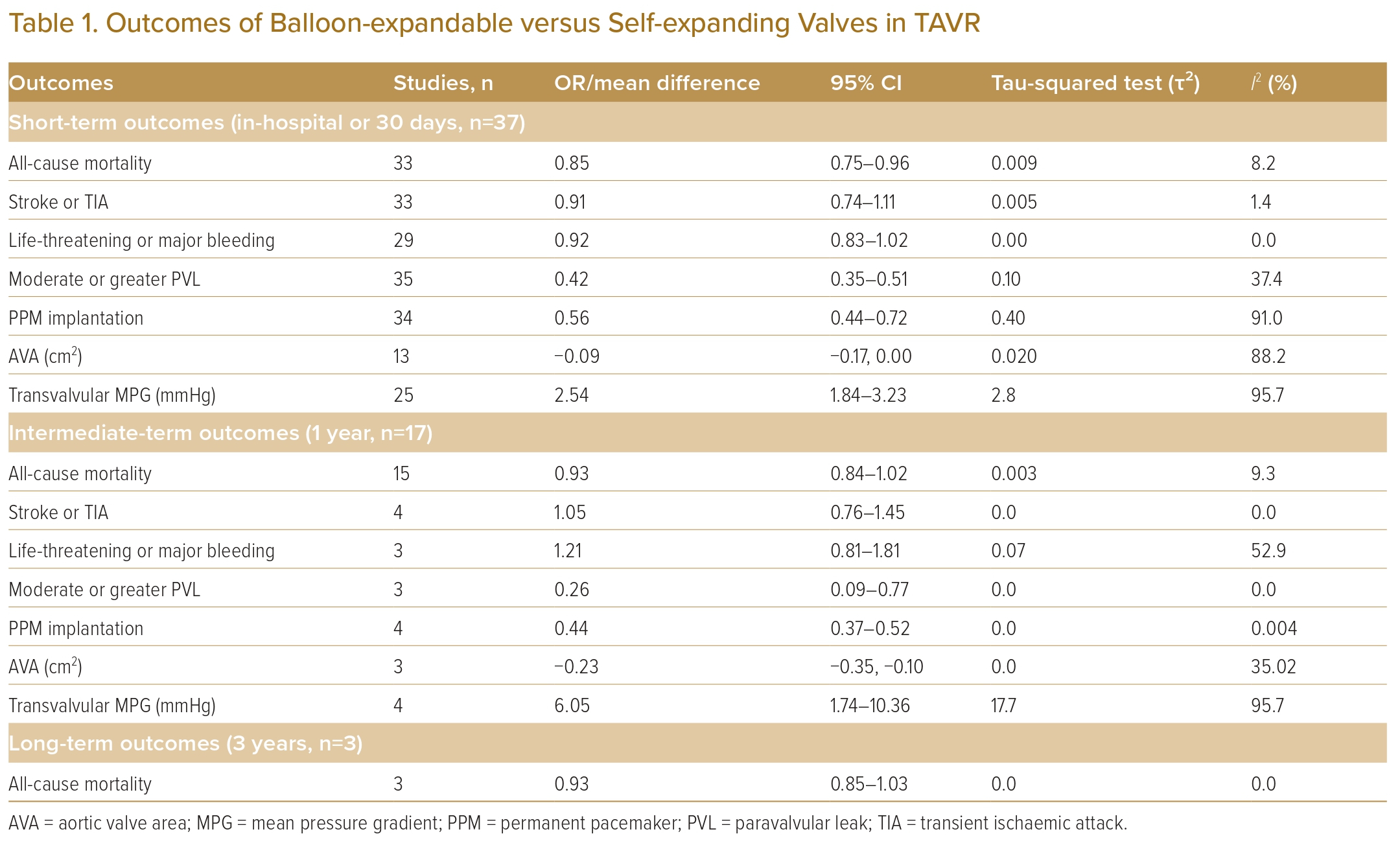
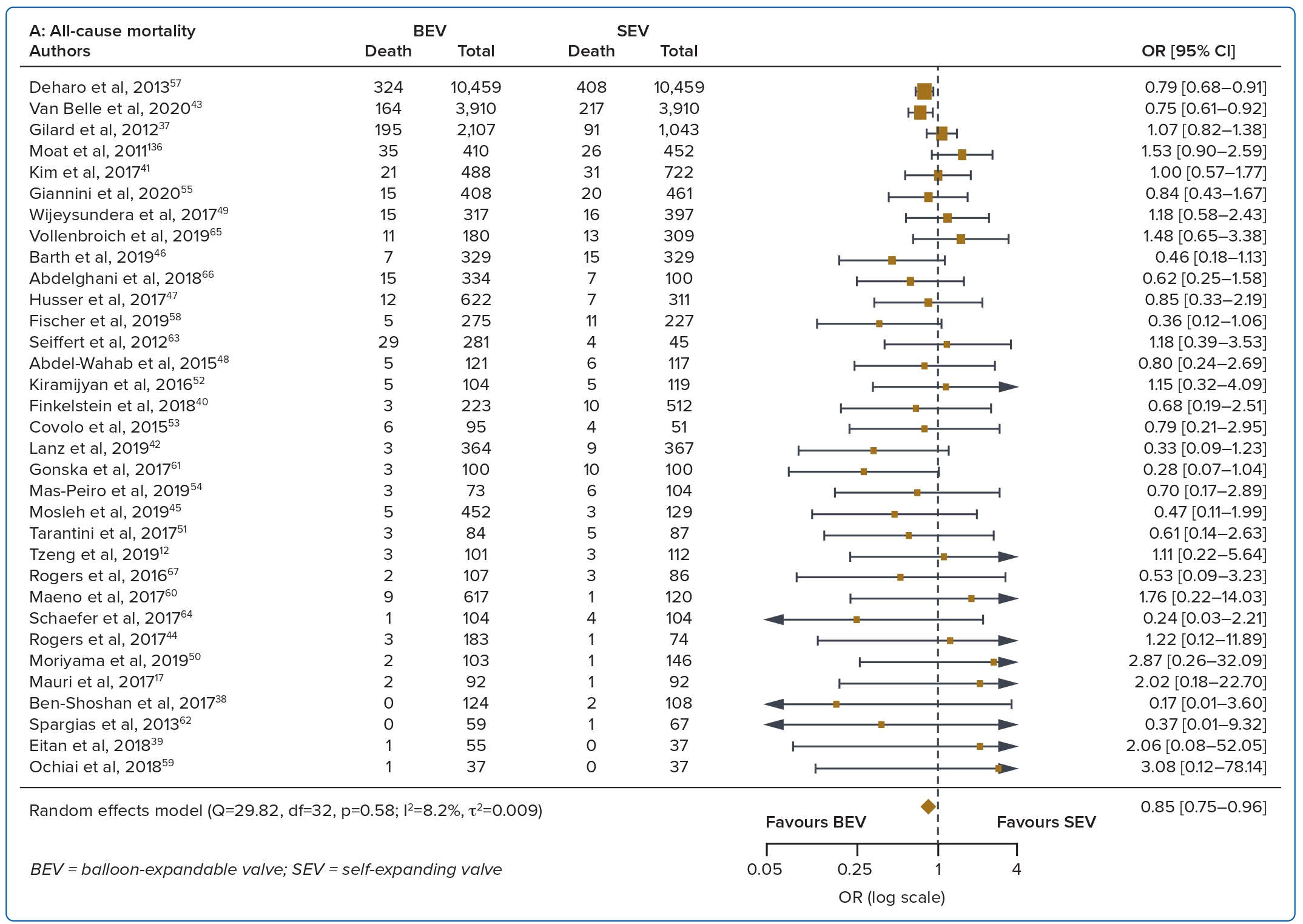
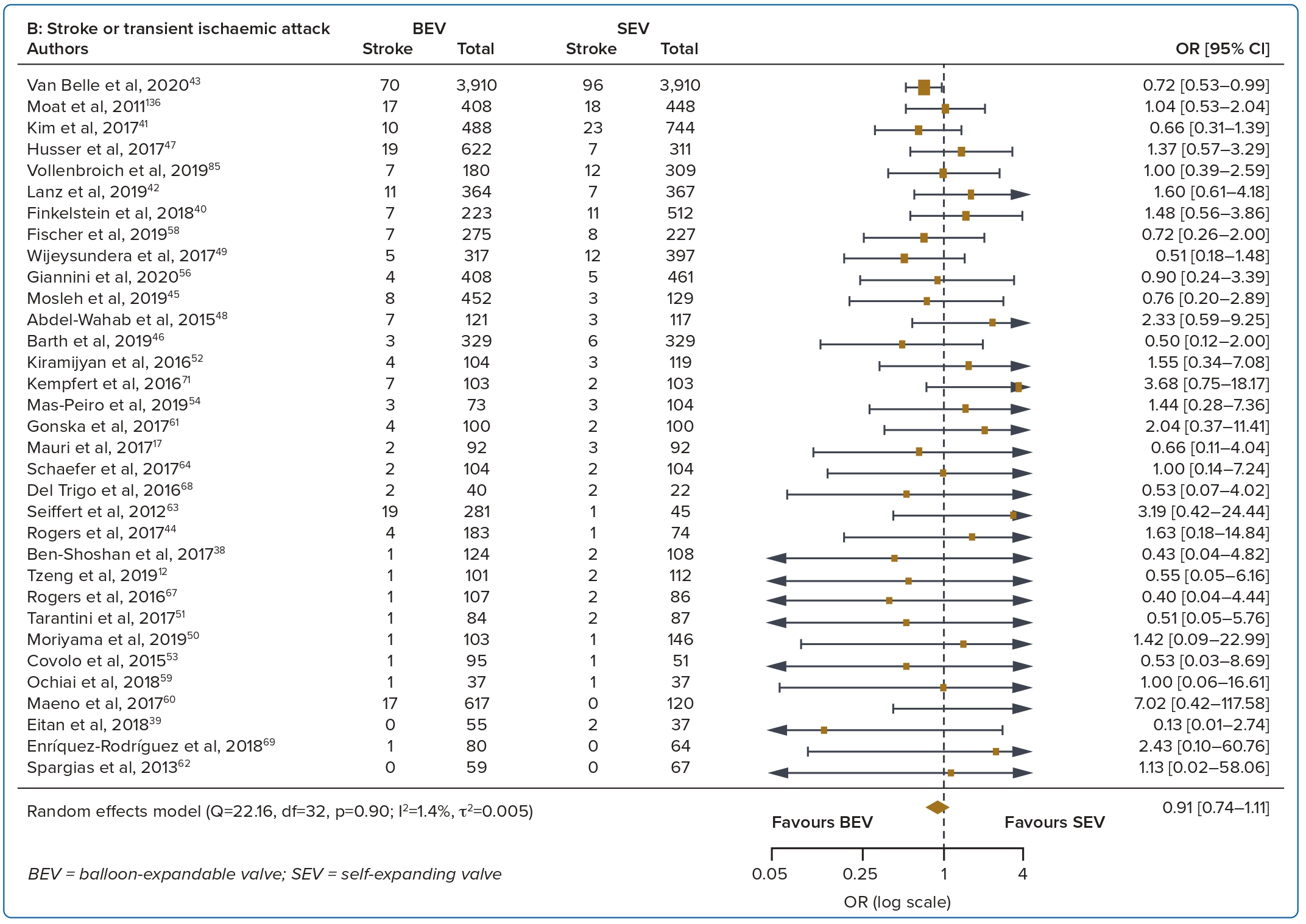
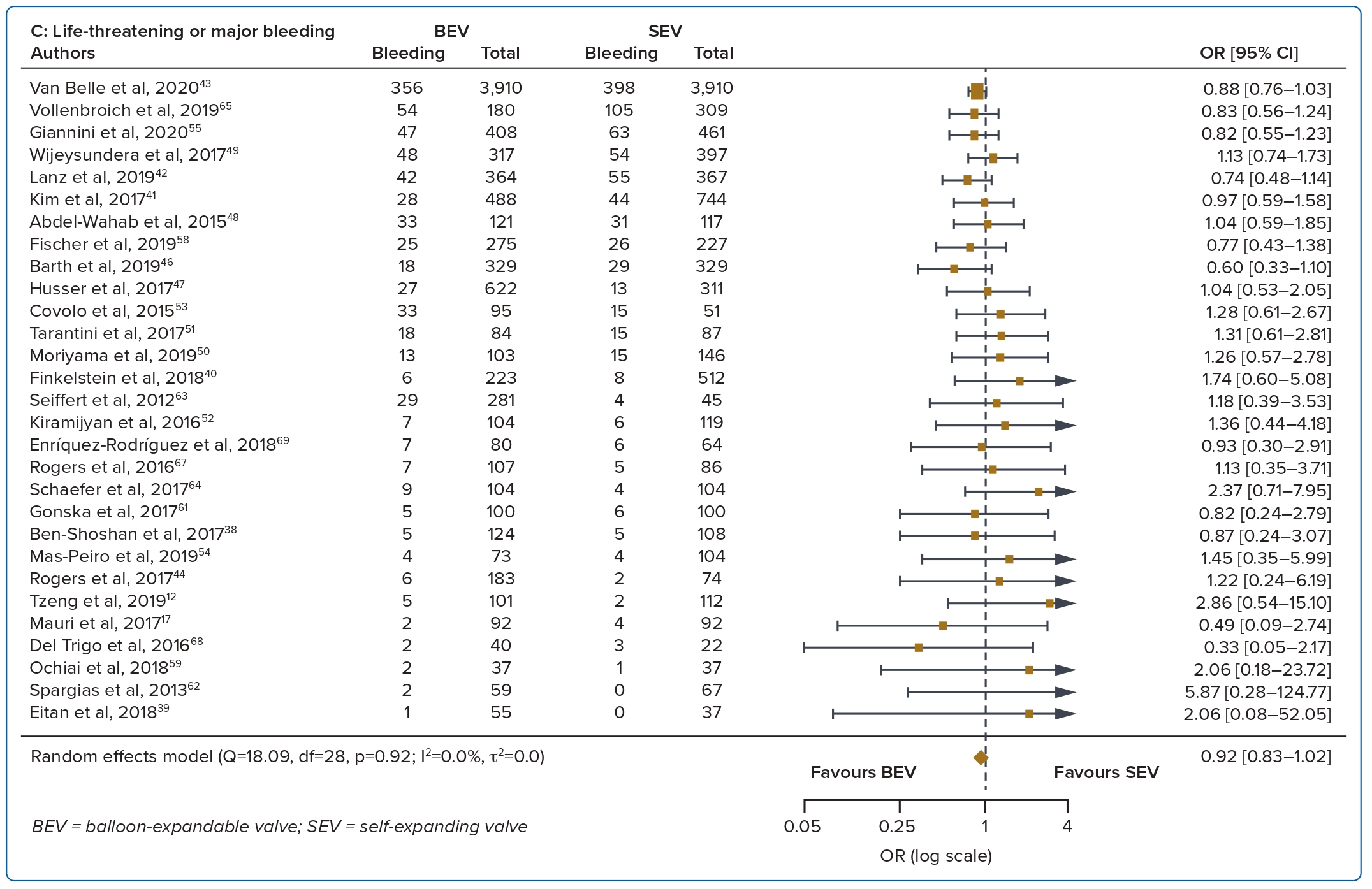
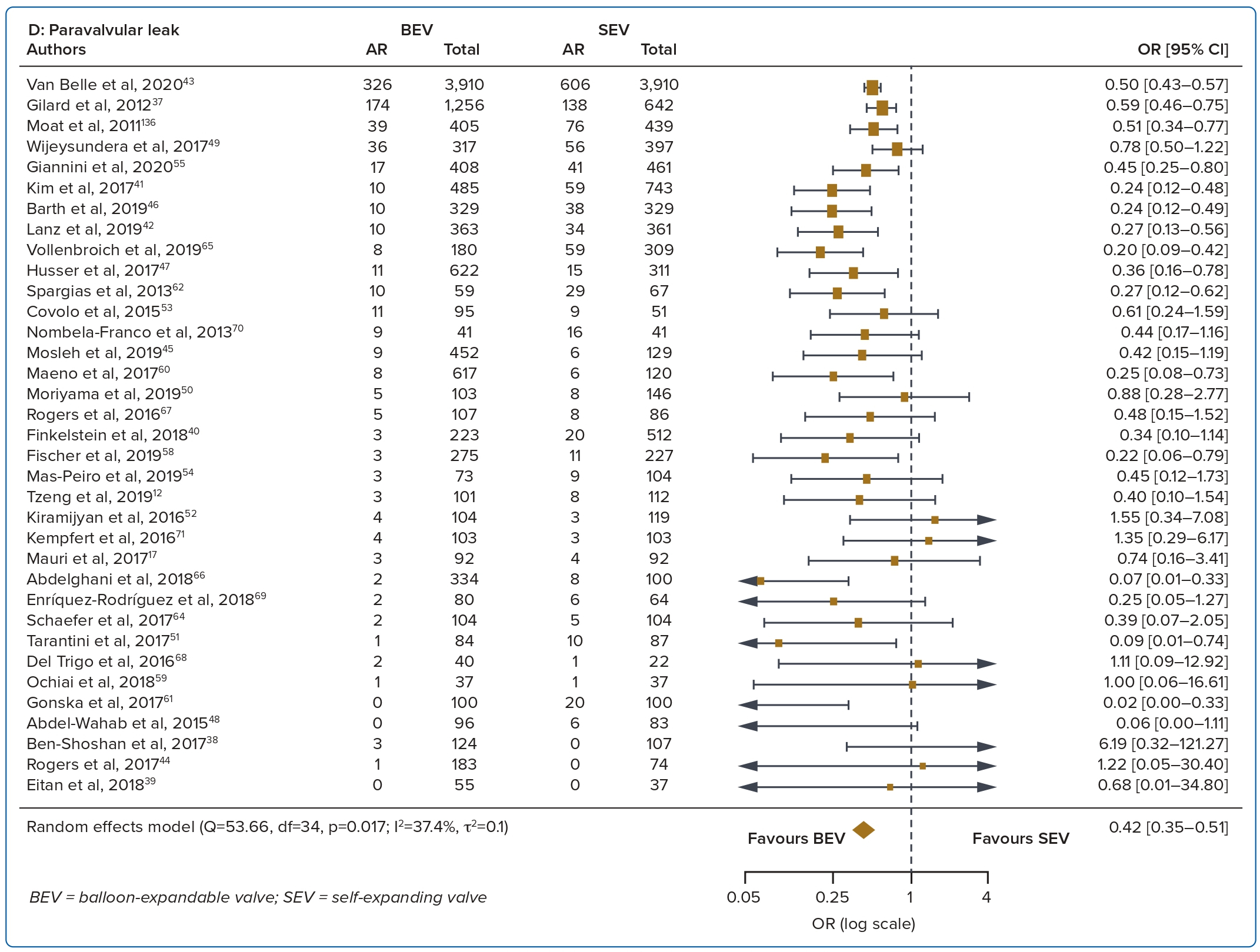
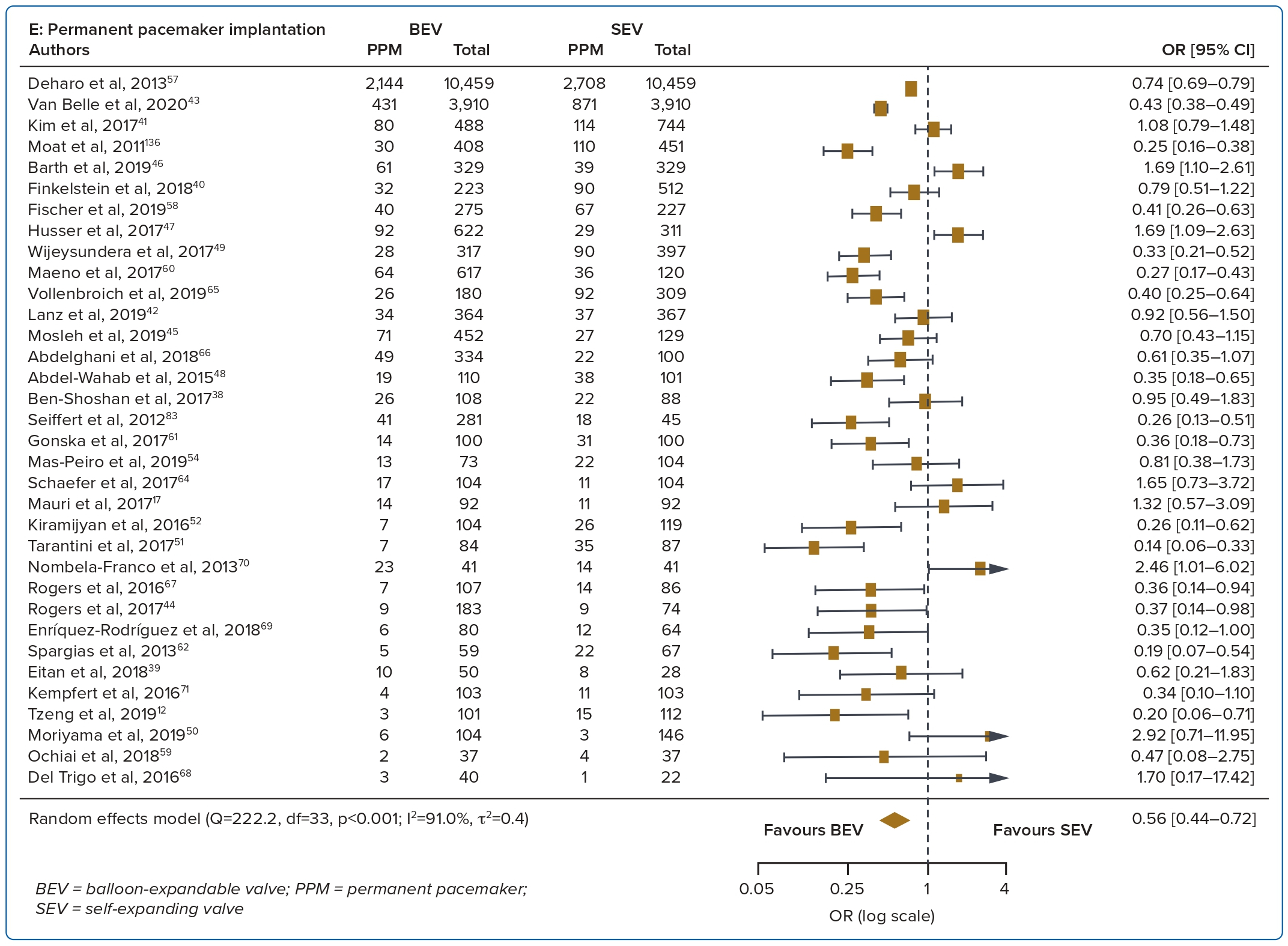
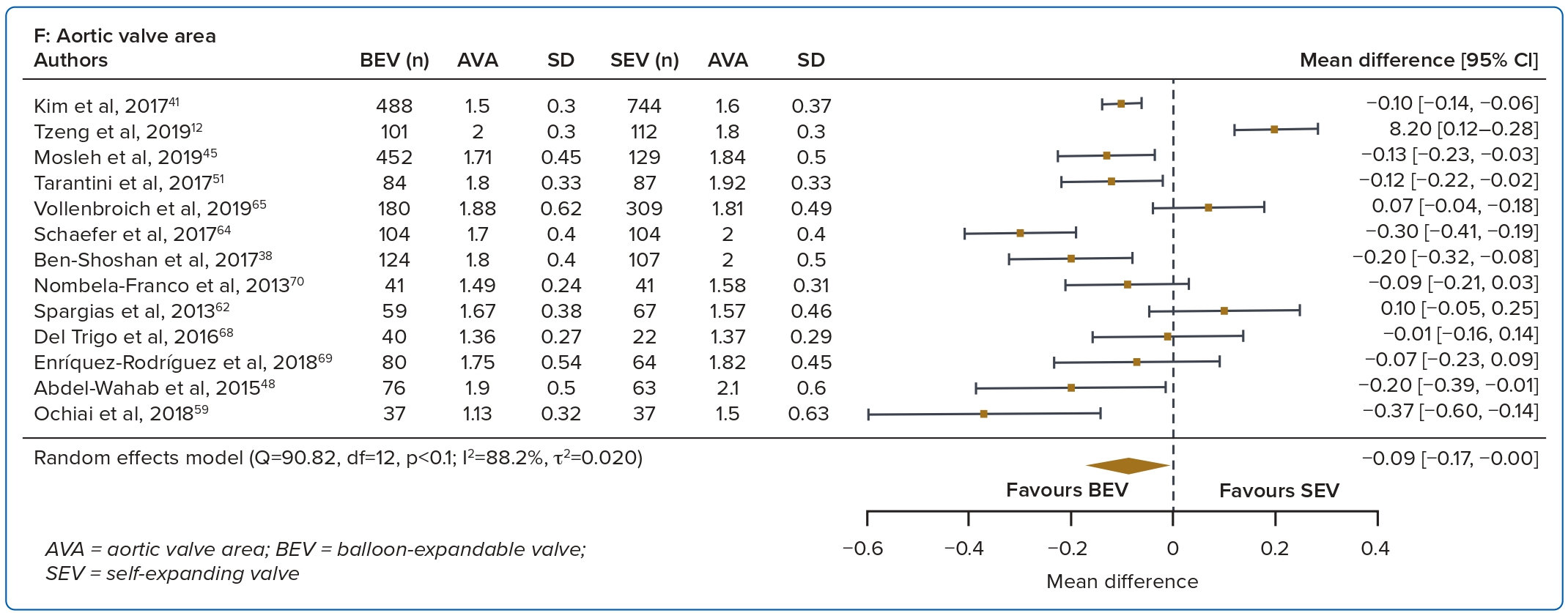
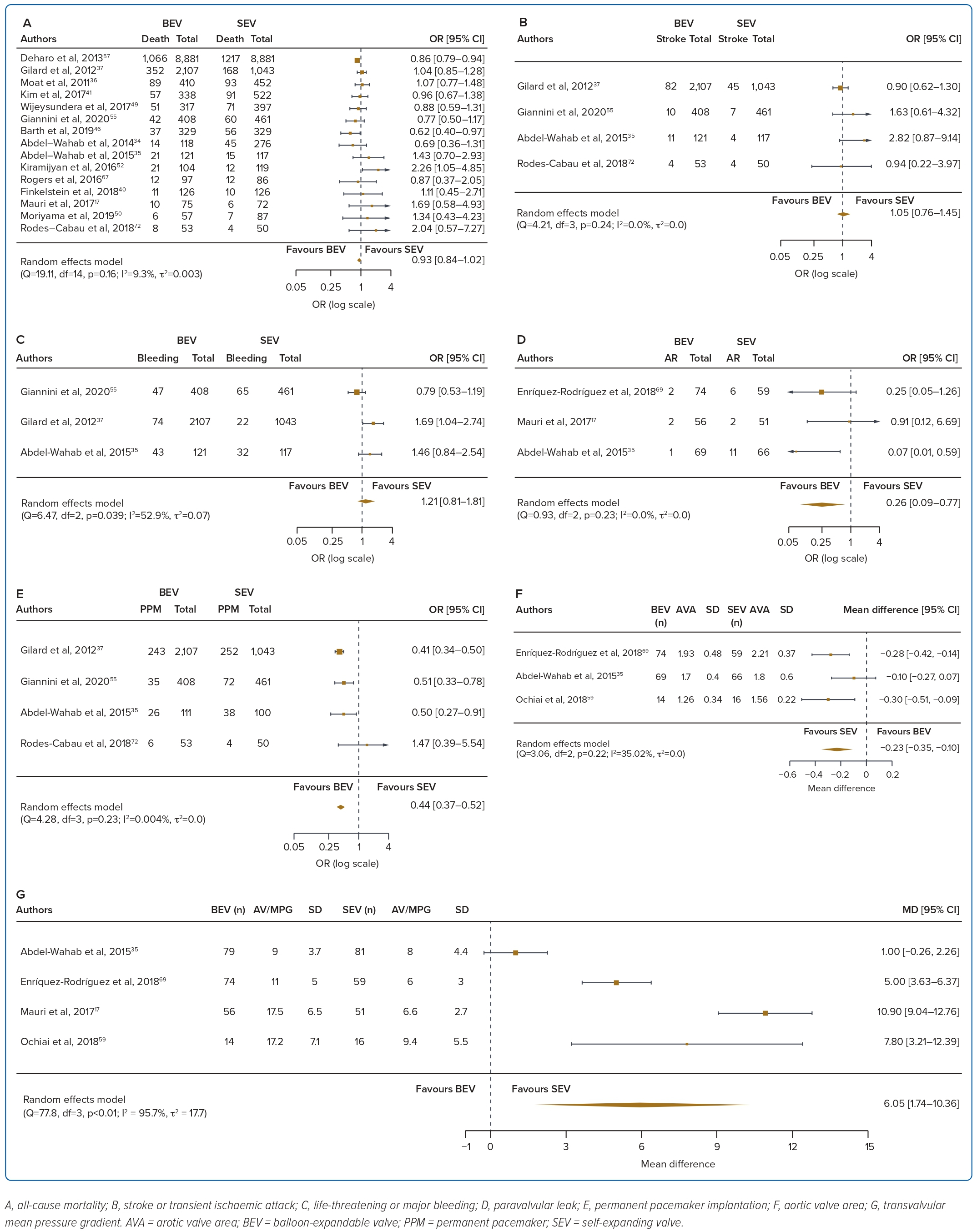
A total of 37 studies (n=45,262) were included. At 30 days, mortality was lower with BEV than SEV (OR 0.85; 95% CI [0.75–0.96]). BEV had lower rates of at least moderate PVL (OR 0.42; 95% CI [0.35–0.51]) and PPM implantation (OR 0.56; 95% CI [0.44–0.72]), but smaller AVA (mean difference −0.09 cm2; 95% CI [−0.17, 0.00]) and higher AV MPG (mean difference 2.54 mmHg; 95% CI [1.84–3.23]). There were no significant differences in stroke/TIA or life-threatening or major bleeding between the two valve designs. Heterogeneity was low for mortality (I2=8.2%), stroke/TIA (I2=1.4%) and life-threatening or major bleeding (I2=0.0%). Heterogeneity was moderate for PVL (I2=37.4%) and high for PPM implantation, AVA and transvalvular MPG (I2>80%) (Table 1, Figure 2 and Supplementary Figure 1A).
Intermediate-term Outcomes (1 year)
A total of 17 studies (n=30,996) were included. At 1 year the BEV design had a lower PPM implantation rate (OR 0.42; 95% CI [0.35–0.51]) and a lower rate of significant PVL (OR 0.26; 95% CI [0.09–0.77]), but a smaller AVA (mean difference −0.23 cm2; 95% CI [−0.35, −0.10]) and higher AV MPG (mean difference 6.05 mmHg; 95% CI [1.74–10.36]). There were no significant differences in mortality, stroke/TIA or life-threatening or major bleeding between the two valve designs. Heterogeneity was low for mortality (I2=9.3%), stroke/TIA (I2=0.0%), PVL (I2=0.0%) and PPM implantation (I2=0.004%). Heterogeneity was moderate for life-threatening or major bleeding (I2=52.9%) and AVA (I2=35.02%), and high for transvalvular MPG (I2=95.7%) (Table 1, Figure 3 and Supplementary Figure 1B).
Long-term Outcomes (3 years)
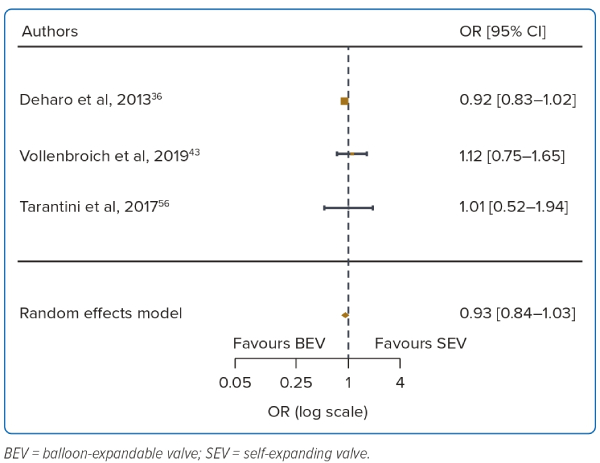
A total of 3 studies were included (n=22,142). At 3 years there was no difference in mortality between BEV and SEV (OR 0.93; 95% CI [0.85–1.03]). There was no heterogeneity between the studies (I2=0.0%). The other outcomes were not analysed due to the small number of reporting studies (Table 1, Figure 4 and Supplementary Figure 1C).
Discussion
This is the largest meta-analysis to compare short- and long-term outcomes between BEVs and SEVs. With 41 studies and 46,000 patients included, the pertinent findings are as follows: the SEV design had better valve haemodynamics than the BEV design; in the short to intermediate term, SEVs had higher PPM implantation and significant paravalvular regurgitation rates than BEVs; there were no differences between bleeding complications and stroke or TIA between the two valve designs; and with regards to mortality, BEVs had improved short-term mortality, but these differences were not seen in the intermediate to long term.
Valve haemodynamics were better at 1 year with the SEV design. This is in part attributable to valve design, in that the supra-annular position of the CoreValve/Evolut platform provides better flow haemodynamics than the intra-annular position of the BEV.13,14
Prosthetic valves that are small for the patient’s size can impair valve haemodynamics and lead to patient–prosthesis mismatch. The persistently high afterload raises ventricular filling pressures and may offset the benefits of aortic valve replacement by impeding regression of left ventricular hypertrophy and atrial dilatation.15,16 This may potentially lead to reduced improvements in functional capacity, affect valve durability and lead to rehospitalisations.5,14,17 Unlike data from surgical aortic valve replacement, however, the issue of patient–prosthesis mismatch in transcatheter heart valves has been controversial, with more recent studies showing no impact on mortality or rehospitalisations.18,19
The rate of PVL was higher with the SEV design at 30 days and 1 year. The higher incidence of at least moderate PVL with SEVs may be attributed to several aspects of the SEV design, such as the smaller outward radial force of the nitinol frame and the greater angulation between the ascending aorta and left ventricular outflow tract.20 PVL results in reduced left ventricular mass regression and increased left ventricular end-diameter.21 A graded relationship has been found between increasing PVL and mortality, with some reports even suggesting an association between mild PVL and death, although this has been debated.21,22 However, it is important to note that there are other determinants of PVL, including aortic valve calcification, larger aortic annulus dimension and elliptical shape. With improvements in valve design including the addition of a sealing skirt, the incidence of PVL has been reduced in the latest generations of the transcatheter heart valves.23,24
PPM implantation rates were higher with SEV than BEV at 30 days and 1 year. This is similar to the findings of many other studies.2,6 The continued pressure of the SEV on the septum may result in tissue oedema and cause conduction disturbances such as left bundle branch block and complete heart block.25 The longer frame and depth of implantation are also potential contributors to the higher PPM rates seen with SEV.26 With recent improvements in implantation technique including the cusp overlap technique, there has been a reduction in PPM rates with the SEV design.27
Bleeding complications and stroke/TIA were not different between the two valve designs at 30 days and 1 year. Improvements to transcatheter heart valve designs over the years have led to progressively smaller and better delivery systems and sheaths, thus greatly reducing overall bleeding complication rates.7,28 Data on stroke are more conflicting. Several studies show a higher incidence of stroke with BEV than SEV while some show similar stroke rates.29,30 A possible explanation for the higher stroke rate with the BEV design includes rapid ventricular pacing-induced cerebral hypoperfusion during BEV deployment.31 Post-TAVR strokes and silent brain infarcts are associated with post-procedure early cognitive decline, leading to increased mortality and morbidity.32
Of note, recent meta-analyses on newer generation transcatheter heart valves have also reported no significant difference in stroke incidence between BEVs and SEVs, in part due to the reduction in post-dilatations needed to reduce PVL after the addition of sealing skirts.33 This was similar to the findings of the current meta-analysis.
Although there was an initial increase in 30-day mortality with SEVs, there were no differences in mortality at 1-year and 3-year follow-up. One meta-analysis using older generation transcatheter heart valves (SAPIEN/SAPIEN XT, CoreValve) also reported an initial increase in 30-day mortality with the SEV but not 1-year mortality.6 This was attributed to initial device failures and procedural complications such as increased residual aortic regurgitation, frequent mispositioning necessitating valve-in-valve procedures, and a higher rate of valve embolisation.6,34
With limited statistical power resulting from a study population of 241 patients, the CHOICE randomised control trial showed that although BEV had higher device success rates, there was no difference in 1-year mortality outcomes between the two valve designs.35 Subsequent meta-analyses with newer generation transcatheter heart valves reported significantly reduced 30-day and 1-year mortality rates with no significant difference between BEVs and SEVs.7,20,28 This current meta-analysis extends existing knowledge by demonstrating that there were no significant differences in long-term mortality between the BEV and SEV designs. The latest advancements in transcatheter heart valve designs as well as increasing operator experience are likely to have contributed to the reduced complications and improved long-term outcomes for patients with aortic stenosis.23,24
Limitations
Several limitations should be acknowledged when interpreting these findings. First, most of the studies included were observational in nature. The pooling of observation studies with different baseline characteristics and inclusion criteria may introduce a greater probability of bias, thus these comparisons should be interpreted with caution. However, the main outcomes reported here, namely mortality, stroke and bleeding complications, were found to have low between-study heterogeneity, and the findings were consistent with other meta-analyses comparing BEVs with SEVs. Only a few randomised controlled trials were available, and inclusion of data from future randomised controlled trials is warranted for more definitive conclusions.
Second, patients in this pooled analysis were not matched for their baseline characteristics (including valve morphology), which may have introduced confounding. Although we would have preferred to perform a meta-regression with adjustment for confounders, not all studies had reported the same baseline and procedural characteristics, making detailed patient-level adjustment technically unfeasible. Third, there are limited long-term data on the comparison of BEVs and SEVs, with only a few studies being included in the analysis. Fourth, the group of self-expandable valves is heterogeneous with potential differences in valve design. This study’s findings may not be generalisable to all SEVs. And last, comparisons made across generations of transcatheter valves may affect the generalisability of the findings. Ongoing improvements in current generations of valve types and implantation techniques will aim to address the drawbacks of the older valve generations and potentially improve outcomes, which may not be captured by this current meta-analysis.
Conclusion
In the short–intermediate term, SEVs had better valve haemodynamics but had higher rates of at least moderate PVL and PPM implantation than BEVs. Nevertheless, there were no significant differences in intermediate to long-term mortality, stroke or TIA, or bleeding complications between the two valve designs. Having a better understanding of these differences will enable TAVR operators to tailor their valve choice based on individual patient profile.
Click here to view Supplementary Material
Clinical Perspective
- Distinct clinical differences exist between balloon-expandable valves (BEVs) and self-expanding valves (SEVs) used in transcatheter aortic valve replacement (TAVR), but long-term comparative data beyond 1 year are lacking.
- In this meta-analysis, SEVs had better valve haemodynamics but higher paravalvular leak and pacemaker implantation rates than BEVs in the short to intermediate term.
- In the intermediate to long term, there were no differences in mortality, stroke, or bleeding complications between the two valve designs.
- Having a better understanding of these differences will enable TAVR operators to tailor their valve choice to individual patient profiles.