Introduction
Takayasu’s arteritis (TA) is a rare, chronic, large-vessel granulomatous arteritis characterised by adventitial thickening, focal leucocyte infiltration of the tunica media and intimal hyperplasia that affects the aorta and its major branches.1 The inflammatory stage of the disease is treated with corticosteroids and other newer immunosuppressants.
Percutaneous transluminal angioplasty (PTA), more so with stents, was not considered highly effective in TA because of many issues, such as multifocal lesions, disease often involving the ostium and firm, scarred and fibrotic lesions, and because of high inflammatory activity resulting in a high chance of restenosis.2 There are only few studies of PTA of arch arteries: some older studies of balloon angioplasties and subsequent studies using self-expanding peripheral stents (SES).2–8
There are hardly any data on the use of balloon-expandable peripheral bare metal stent (BMS) or coronary drug-eluting stents (DES) in patients with TA, except for a few case reports.9,10 However, logically, because of their ostial nature and negative remodelling resulting in a smaller diameter, these lesions should be better suited for balloon-mounted stents, namely coronary DES or peripheral BMS.
Therefore, we undertook this prospective longitudinal study to determine the initial success and the 6-month restenosis rate in arch vessel angioplasty in TA patients using SES or balloon-expandable stents (peripheral BMS or coronary DES), as required, as well as the factors associated with restenosis.
Methods
The study was conducted at Govind Ballabh Pant Institute of Post Graduate Medical Education & Research (GIPMER), New Delhi, from April 2016 to October 2017. All consecutive patients diagnosed with TA were evaluated and 18 symptomatic patients with aortic arch vessel involvement suitable for angioplasty were selected. TA was diagnosed as per the American College of Rheumatology (ACR) criteria.11 Informed consent was obtained from all patients. We followed the ethical standards of human experimentation (institutional and national) and the study was performed in accordance with the Helsinki Declaration of 1964. The study was approved by the Institutional Review Board of GIPMER.
Baseline demographics, symptomatology and laboratory parameters for all participants are presented in Supplementary Table 1.
Patients underwent angiography of the aortic arch and abdominal aortography via femoral access under local anaesthesia. Selective angiography of the stenosed artery was performed to localise the site and extent of vessel involvement and distal circulation.
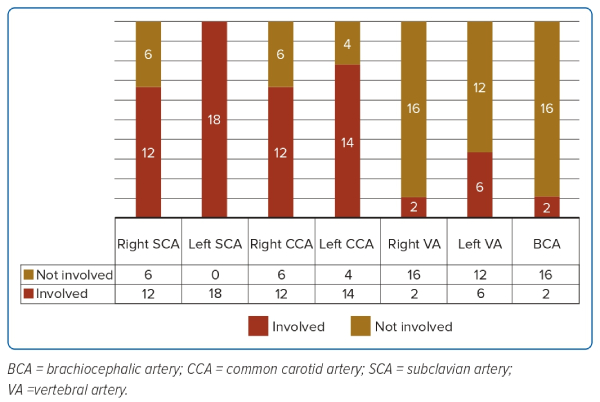
The pattern of arch vessel involvement is detailed in Figure 1 and Supplementary Table 2.
Angioplasty was not attempted in vessels with total occlusion. Angioplasty was attempted in the following cases:
- symptomatic patients; and
- asymptomatic patients with >70% stenosis.
Angioplasty was performed according to standard protocols. Angioplasty was performed by a group of operators with more than 20 years of experience in peripheral angiography and angioplasty, including arch vessel and aortic interventions, and nearly 100 interventions in TA patients. All patients were given aspirin 150 mg/day and clopidogrel 150 mg/day for at least 3 days before the intervention. After the procedure, aspirin 150 mg/day and clopidogrel 75 mg/day was continued for 6 months. Thereafter, only aspirin 150 mg/day was continued. Patients with evidence of disease activity, as suggested by an increased ESR, were given oral steroids/methotrexate.
We deployed SES for the treatment of lesions in carotid or subclavian arteries. Conversely, we used coronary DES mostly in the case of vertebral artery involvement, for ostial placement in the case of carotid or subclavian artery involvement or where there was severe negative remodelling of the vessel due to diffuse disease. Peripheral BMS were used mostly in ostial lesions with a diameter larger than the available DES.
Quantitative Angiography
Quantitative angiography was performed using a General Electric Dicom Centricity in Advantage Workstation by a single cardiologist, with more than 5 years of experience in quantitative angiography, who was blinded to the patients’ clinical details. The Stenosis Analysis PCI Assist application was used to quantify the lesion. Once the image had been acquired and digitally processed, computer manipulation was performed. The most critical component of the computational analysis was the algorithm of boundary delineation within the area of interest. This method required identification of the artery segment to be analysed and accurate computer-assisted vessel edge delineation. To derive quantitatively useful information from the artery segment analysis, a calibration function converted measured pixels to in vivo millimetres, using reference standards derived from the contrast-filled catheter. Using the image obtained from the contrast angiogram, the minimum lumen diameter (MLD) was measured and compared to the diameter of the ‘normal’ vessel immediately adjacent, which served as the reference diameter (RD; Supplementary Figures 1 and 2). Percentage stenosis was then calculated using the equation (1 − MLD/RD) × 100.
Angiographic Success
We measured MLD immediately after the angioplasty. Angioplasty was considered technically successful if the residual stenosis was less than 20% of the lumen diameter with at least a 50% increase compared with the pretreatment diameter.
Procedural Success
Procedural success was assessed on the basis of technical success and the absence of procedure-related complications.
Follow-up
Patients were followed up for 6 months after treatment. We did diagnostic angiography in all patients at 6 months. We measured MLD and percentage stenosis in all lesions, and in-stent restenosis (ISR) and lumen loss at 6 months were calculated at 6 months by comparing the MLD at 6 months and with that immediately after angioplasty. Net lumen gain was measured by comparing MLD at 6 months with preprocedural MLD. During follow-up, patients were also assessed for symptoms.
Statistical Analysis
Statistical analyses were performed using SPSS software 29.0.1.0.
Quantitative variables were compared using Student’s t-test and paired t-test, as appropriate, whereas qualitative variables were compared using the Chi-squared test. Where appropriate, quantitative variables are presented as the mean ± SD. Correlations between quantitative percentage restenosis and different variables were evaluated using Pearson’s correlation.
Results
Angioplasty Procedural Details
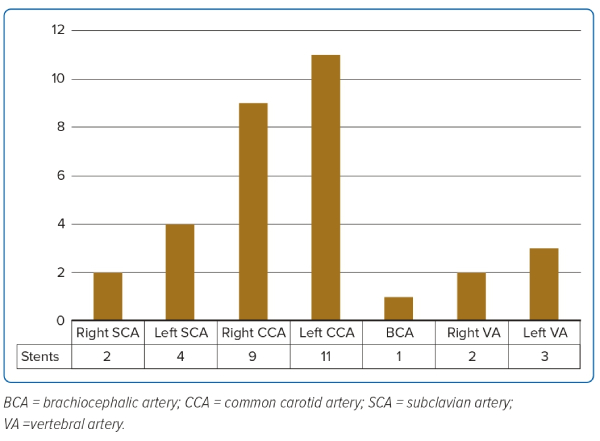
Altogether 29 lesions were addressed in 18 patients. As shown in Figure 2, the most commonly stented artery was the left common carotid artery, followed by the right common carotid artery and left subclavian artery (12.5%). Altogether 32 stents were deployed, 15 SES, 14 coronary DES and three BMS (for details, see Supplementary Table 3).
Immediate Angiographic Success with SES
Twelve patients were treated with 15 SES. All patients experienced angiographic and procedural success. The SES had a diameter of 7 mm or 8 mm and ranged in length from 37 to 100 mm. Based on quantitative angiographic analysis, the preprocedural MLD was 1.0 ± 0.6 mm and percentage stenosis of the lesions was 81.0 ± 9.6%. As indicated in Supplementary Table 4, there was an immediate mean lumen gain of 2.95 ± 1.1 mm and mean lesion percentage stenosis decreased from 81.0% to 20.2%, both these changes were highly statistically significant.
Sustained Angiographic Success with SES
Check-up angiography was performed at the 6-month follow-up. However, two patients were lost to follow-up, so 10 patients with 13 stents could be profiled at 6 months. All the angiograms were again analysed quantitatively, with some representative angiograms shown in Supplementary Figure 3.
We compared the MLD at the 6-month follow-up with the MLD before stenting and after angioplasty in the case of these 13 stents (Supplementary Table 5). Although there was a lumen loss of 2.01 ± 1.13 mm over 6 months, the MLD increased from 1.04 ± 0.63 mm at baseline to 1.98 ± 1.33 mm at 6 months (p=0.013, paired t-test), giving a mean lumen gain of 0.94 ± 1.16 mm. In fact, there was a net lumen loss at 6 months in only two patients.
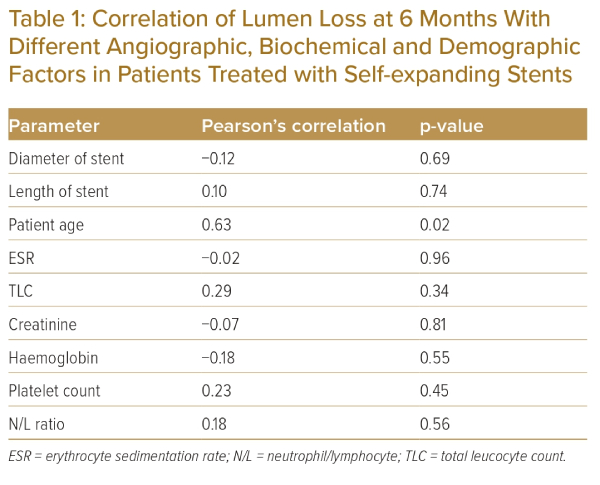
Pearson’s correlation analysis for associations between different demographic, biochemical and angiographic parameters and lumen loss over the 6 months after angioplasty revealed a significant negative correlation between lumen loss and the age of the patient. However, there were no correlations between lumen loss and disease activity markers (e.g. ESR, total leucocyte count, neutrophil/lymphocyte ratio or platelet count) or the diameter or length of the stent (Table 1).
Symptom Assessment in Patients Treated with SES
All 10 patients experienced improvement after the angioplasty that was mostly sustained up to 6 months. In fact, patients with significant ISR were also asymptomatic at follow-up.
Immediate Angiographic Success with Coronary DES
Eight patients were treated with 14 DES. DES varied in diameter from 3.5 to 4.5 mm and in length from 13 to 38 mm. All patients experienced angiographic and procedural success. Based on quantitative angiographic analysis, the preprocedural MLD was 1.0 ± 0.5 mm and lesion percentage stenosis was 79.6 ± 9.5%. As indicated in Supplementary Table 6, there was an immediate mean lumen gain of 2.1 ± 0.7 mm and mean lesion percentage stenosis decreased from 79.6% to 20.5 %, with both these changes being highly statistically significant.
Sustained Angiographic Success With Coronary DES
Angiography was performed at the 6-month follow-up. However, two patients were lost to follow-up. Altogether, six patients with 11 stents could be profiled at 6 months. All the angiograms were again analysed quantitatively, with some representative angiograms shown in Supplementary Figure 4.
We compared the MLD at the 6-month follow-up with that before stenting and after angioplasty for these 13 stents (Supplementary Table 7). Although there was a lumen loss of 1.6 ± 0.8 mm over 6 months, the MLD increased from 0.94 ± 0.17 mm at baseline to 1.34 ± 1.16 mm at 6 months (p=0.317, paired t-test), giving a mean lumen gain of 0.40 ± 1.26 mm. In this group, two patients with left vertebral artery ostial stenting had 100% restenosis at 6 months.
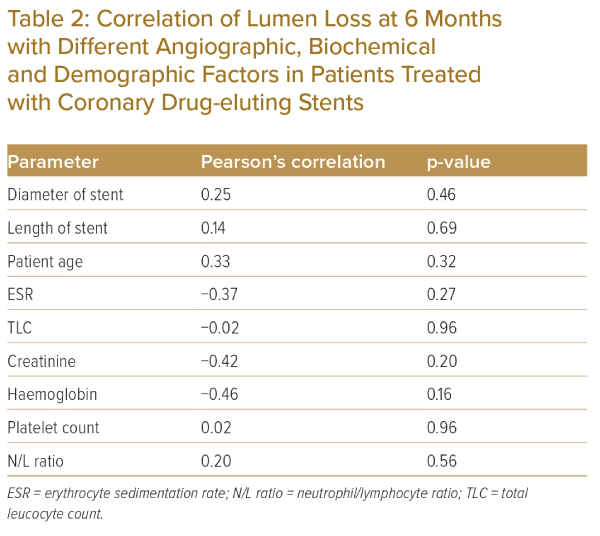
Pearson’s correlation analysis of the association of different demographic, biochemical and angiographic parameters with lumen loss over 6 months after angioplasty revealed no correlations between any of the factors and lumen loss in the case of coronary DES (Table 2).
Symptom Assessment in Patients Treated with Coronary DES
All six patients experienced improvement after angioplasty that was mostly sustained up to 6 months, except for the two patients with vertebral artery ostial 100% restenosis, who became symptomatic with episodes of syncope.
Initial Procedural Success and Percentage Restenosis in Coronary DES Versus SES
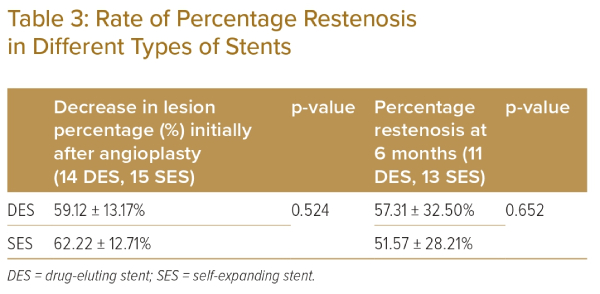
Because the DES had a narrower diameter than the SES, these could be compared only by means of acute decrease in lesion percentage stenosis and percentage restenosis. As evident from Table 3, both SES and DES had similar acute procedural success rates and similar percentage restenosis rates at 6 months.
Angiographic Success With Balloon-Expandable Peripheral BMS
Three patients had a balloon-expandable peripheral BMS placed. These were mostly used for ostial lesions in large-diameter carotid arteries.
One patient had a 6 mm × 14 mm stent placed in the ostium of the right subclavian artery. In this case, there was an acute lumen gain of 3.78 mm (from 2.13 to 3.91 mm) and, after 6 months, there was in-stent restenosis of 51.15% with a lumen loss of 2.00 mm. There was a net lumen loss of 0.62 cm. A second patient had a 6 mm × 18-mm stent placed in the ostium of the left common carotid artery. There was acute lumen gain of 2.71 mm (from 1.07 to 3.78 mm) and, after 6 months, there was in-stent restenosis of 55.29% with a lumen loss of 2.09 cm. The net lumen gain was 0.62 cm. In the third patient, the 6-mm × 18-mm stent was placed in the ostium of the left common carotid artery. There was an acute lumen gain of 2.60 mm (from 0.60 to 3.20 mm).
Discussion
This study is the first prospective study specifically intended to determine the results of angioplasty in aortic arch vessels in TA patients. There is only very limited data regarding angioplasty of arch vessels in patients with TA using SES or DES. Bali et al. described successful PTA in eight TA patients but they did not describe any follow-up of these patients.4 Singh et al. described PTA in TA and, in their series, five patients underwent successful PTA of the left subclavian artery, with stent implantation in four patients.5 There were no complications, with symptom relief seen in all patients postoperatively. All patients had a well-palpable pulse on follow-up. Liang et al. undertook a retrospective analysis of the Cleveland Clinic Foundation’s experience in providing PTA for TA and reported that three of seven balloon angioplasties and five of seven stents restenosed or became occluded after 1–72 months and 2–45 months of follow-up, respectively.6 In the retrospective cohort study of Labarca et al. of patients with TA who underwent vascular interventions at a tertiary centre between 1984 and 2009, there were nine subclavian, one carotid and one vertebral artery stents placed.7 In that study, the restenosis rate was 55.56% at 6.2 years.7 In the study of Saadoun et al., there were five subclavian and four carotid angioplasties, with a reported restenosis rate of 75.7% at 6 years.8
In the present study, altogether 29 lesions were treated by angioplasty in 18 patients. Conventionally, peripheral stents, either SES or balloon-expandable BMS, are used for carotid or subclavian arteries. This is the first prospective study reporting the use of coronary DES to treat lesions of peripheral arteries.
Immediate angioplasty results were excellent in all patients and were comparable for peripheral stents and coronary DES, with no procedure-related complications or mortality in either group. However, despite there being lumen loss at the 6-month follow-up in both groups, overall we achieved a significant lumen gain of 0.94 ± 1.16 mm with SES and 0.40 ± 1.26 mm with coronary DES at 6 months compared with the initial pre-angioplasty lumen diameter. This was corroborated by the fact that there was persistent and significant symptom relief at 6 months. In fact, patients were mostly asymptomatic, even after significant restenosis. This may indicate that whatever lumen gain was sustained at 6 months was sufficient for symptom relief. Therefore, in TA patients, the primary treatment objective should be the clinical improvement, rather than only targeting cosmetic angiographic results.
When we examined correlations between lumen loss at 6 months and different demographic, biochemical and angiographic parameters, we found a significant negative correlation between lumen loss and patient age for lesions treated with SES. In TA, there is a significant association between the age of the patient and disease activity and severity.1 Patients who were older were mostly patients without active disease of the disease, thus having a lower rate of restenosis.
Similarly, we investigated factors responsible for lumen loss in the case of coronary DES, but we failed to find any correlation between restenosis and any demographic, angiographic or procedure-related factors. Overall, coronary DES had results comparable to that of peripheral SES. Although the observed incidence of ISR in coronary lesions with DES is less than 10%, the rate of restenosis in peripheral artery lesions with these stents has not been explored to date.12,13 There are only few case reports of DES being used in carotid or subclavian arteries in TA patients.9,10 We used coronary DES in small-calibre vessels, like vertebral arteriesm, or in cases where there was severe negative remodelling due to highly active disease with the assumption that there will be less restenosis with DES due to antiproliferative drugs. The restenosis rate in our series may look very high, but we need to consider the fact that all these were highly symptomatic patients with very active disease. We also need to consider that these lesions were mostly ostial lesions, and that TA per se is a highly destructive disease. In fact, the mechanism of artery stenosis in TA is different than that in atherosclerotic coronary lesions. In TA, the basic pathology is that of chronic intramural inflammation, which leads to significant restenosis.9 In addition to typical in-stent stenosis, external stent compression by progressive vessel wall fibrosis and calcification have also been described.9
DES can be used as a treatment option in carotid and subclavian ostial lesions, or in case of vertebral artery lesions. In fact, these lesions are challenging to treat with SES because ostial placement is not easy with these stents and there is always a chance of stent migration. In these situations, coronary DES are viable options, especially because we have shown here that they produce results comparable to SES. Another important fact to be considered here is that in TA, because of destructive disease, the artery diameter becomes significantly narrower, so these smaller stents, with a diameter between 3.5 and 4.5 mm, may be ideal in many of these patients.
It was observed that three patients had DES placed in the ostium of the left vertebral artery, two of whom had 100% restenosis and the other having 64.54% restenosis, suggestive of significant ISR. There are very limited data available regarding restenosis rates in the case of vertebral artery stenting. Albuquerque et al. and Ko et al. have reported restenosis rates as high as 43.3% and 30.8% in these lesions, but these cohorts consisted mostly of older patients with atherosclerosis.14,15 With TA, we expect a higher rate of restenosis, but no such series is available. Left vertebral ostial disease is a bifurcation lesion with disease in both the pre- and post-vertebral subclavian artery. In such cases we performed isolated side branch (vertebral) angioplasty to increase the cerebral blood flow for intractable syncope. However, with time, the subclavian artery disease progressed, leading to critical or complete occlusion of the side branch (i.e. vertebral artery). This scenario is similar to isolated ostial left circumflex (LCX) stenting, where left main to left anterior descending artery disease progresses and finally causes ostial LCX ISR.
In the present study, the patients were mostly in the active stage of the disease, as indicated by a mean ESR of 40.55 ± 21.02 mm/h. However, there was no correlation between ESR and ISR in either of the two groups in our study. In fact, there was no correlation between ISR and other activity markers (e.g. platelet count, neutrophil/lymphocyte ratio, C-reactive protein). This may be because of either the small sample size or the fact that disease activity in the whole cohort was higher, thus masking any correlations.
Overall, TA is a catastrophic disease with significant morbidity because of arch vessel, mainly subclavian and carotid, involvement. Despite disease-modifying agents, the overall prospect for TA patients remains bleak. Surgical bypass may be the last option in some patients, but endovascular intervention is always a ray of hope. To date, there have been no prospective studies specifically following up of angioplasty in arch vessels in TA patients. In fact, no study to date has tried to quantitatively define lesions as well as ISR in these patients or has objectively tried to identify factors correlated with ISR in TA patients. Our group of TA patients had significant disease activity at the time of stent placement; however, all the patients had highly symptomatic disease and stenting resulted in significant symptom relief.
We would like to highlight that although endovascular intervention is feasible in TA with arch vessel involvement, it comes at the cost of restenosis. So, it should only be offered to patients with severe persistent symptoms like syncope, loss of vision or persistent rest claudication, and preferably after controlling disease activity. Although the follow-up results are not very gratifying because of the high rate of restenosis, the persistent symptom improvement provides a better quality of life for patients with such a difficult-to-treat disease.
Our study could pave the path for future larger studies with longer follow-up in TA patients and, simultaneously, this may encourage interventionalists to explore endovascular treatment in subclavian and carotid vessels in this specific group of patients, thus benefiting more patients. 
Click here to view Supplementary Material
Clinical Perspective
- Takayasu’s arteritis (TA) with arch vessel involvement is an aggressive disease with very poor long-term prognosis.
- Drug-eluting stents and self-expandable stents could be used to treat TA patients to get good initial angiographic results.
- Although there is considerable restenosis of these stents at 6 months, there is good lumen gain compared with initial pre-angioplasty results for the same vessels.
- The overall lumen gain results in good symptom improvement in TA patients.