Right ventricular outflow tract (RVOT) dysfunction in the adult congenital heart disease (ACHD) population frequently requires repeated surgical interventions throughout the patients’ lives, with significant associated morbidity.1 Percutaneous pulmonary valve implantation (PPVI) was developed as an alternative to open heart surgery, and was first performed by Bonhoeffer et al. in 2000.2 Since then, several devices have been engineered for PPVI, including the Melody valve (Medtronic) and the Edwards Sapien S3 valve (Edwards Lifesciences).3–10
PPVI has been proven to normalise high RVOT gradients and treat pulmonary regurgitation (PR), ameliorating symptoms and improving effort tolerance, as well as improving right ventricle dimensions, haemodynamics and systolic function.5–8,11,12 This allows right ventricle reverse remodelling, which has been associated with improved left ventricular (LV) filling and systolic function.12 To date, PPVI is being used increasingly worldwide, and this article explores our early local experience with PPVI, as well as the necessary precautions to be taken and the management of associated complications.
Methodology
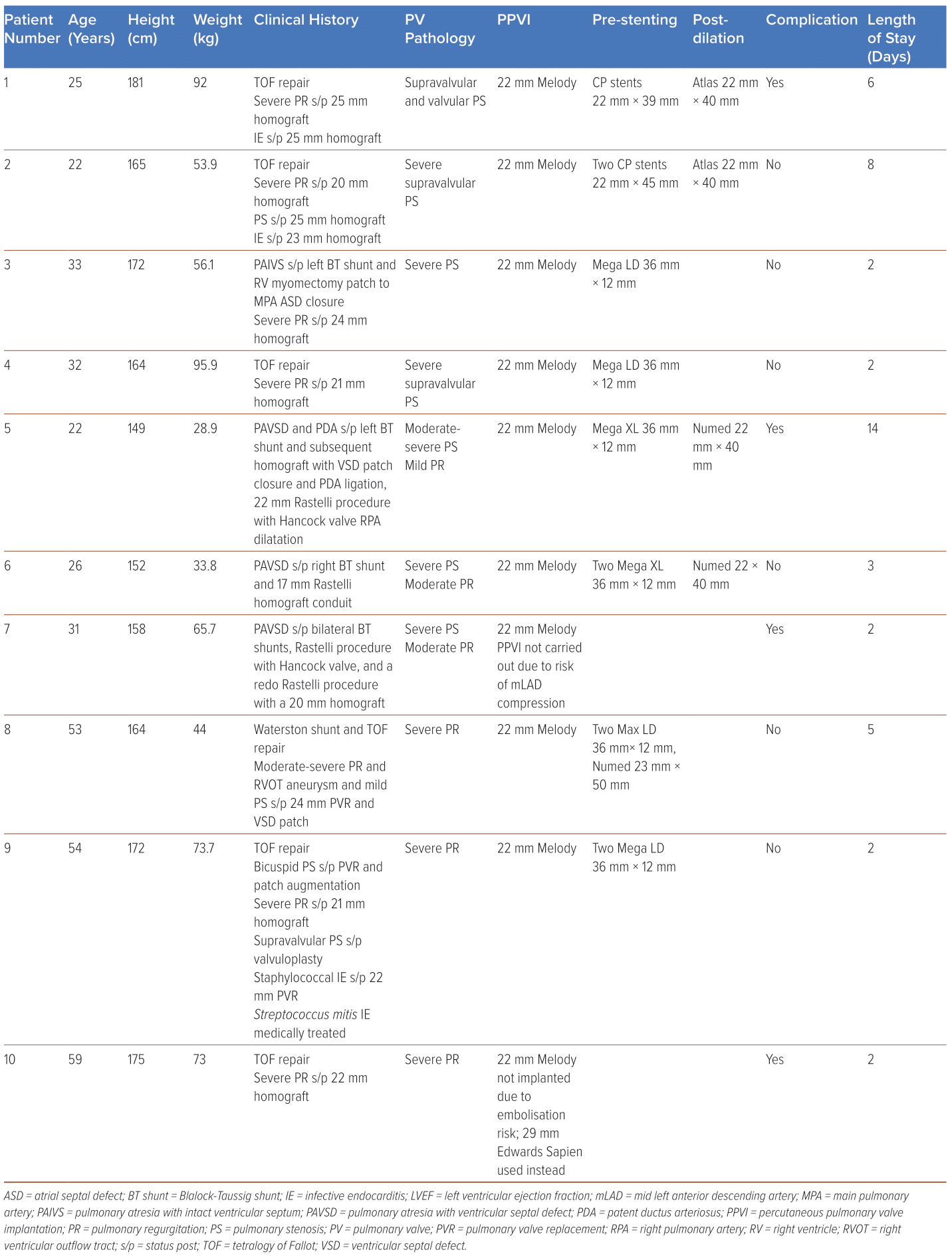
Between 2017 and 2022, PPVI was attempted in 10 patients with ACHD at a single local tertiary centre (Table 1). All had undergone previous surgical intervention to the RVOT with subsequent severe pulmonary stenosis (PS; n=6) or severe PR (n=4), and were recommended for PPVI based on the American Heart Association and European Society of Cardiology ACHD guidelines on indications for pulmonary valve intervention.13,14
Patients underwent multimodality imaging with transthoracic echocardiography (TTE), CT scans, cardiac MRI and coronary angiography to determine their suitability for PPVI. In particular, TTE was used to examine pulmonary valve function including measures of Vmax, peak pressure gradient (PPG) and mean pressure gradient (MPG), right ventricle dimensions and function including tricuspid annular plane systolic excursion (TAPSE) and tricuspid annulus S’ (TA S’), tricuspid regurgitation and pulmonary artery systolic pressure. Additionally, all patients underwent dental assessment and clearance before the procedure.
PPVI with a Melody valve was then performed electively in the cardiovascular laboratory under fluoroscopic guidance and general anaesthesia. Femoral vascular access was obtained under ultrasound guidance. Routine balloon testing was carried out to rule out the risk of coronary compression by PPVI. All patients underwent pre-stenting with one or two stents, after which PPVI was performed, and post-dilatation carried out in two of the 10 patients to improve valve expansion and stability. Femoral vascular access was then closed with Perclose ProGlide devices (Abbott) and manual compression applied. Of note, all patients experienced a reduction in pullback gradient, and improvements in right ventricular (RV) and pulmonary artery (PA) pressures.
Patients underwent regular clinical follow-up for a mean of 3.1 ± 1.7 years, including serial TTEs on post-procedure day 1, at 6 weeks, at 6 months and yearly thereafter to assess valve and ventricular function, as well as repeat chest X-rays to assess valve stability and identify complications such as stent fractures. No reintervention was required in all nine patients who received successful PPVI, and there have been no deaths so far during follow-up. Patients were continued on lifelong single antiplatelet therapy and the importance of dental hygiene was reinforced to decrease the risk of infective endocarditis (IE).
Results
On-label Melody PPVI implantation had an 80% success rate in 10 ACHD patients (mean age 35.8 ± 13.7 years; 60% male). An Edwards Sapien S3 PPVI was used instead of Melody PPVI in patient #10 in view of large RVOT size, and Melody PPVI was not carried out in patient #7 due to the risk of coronary compression.
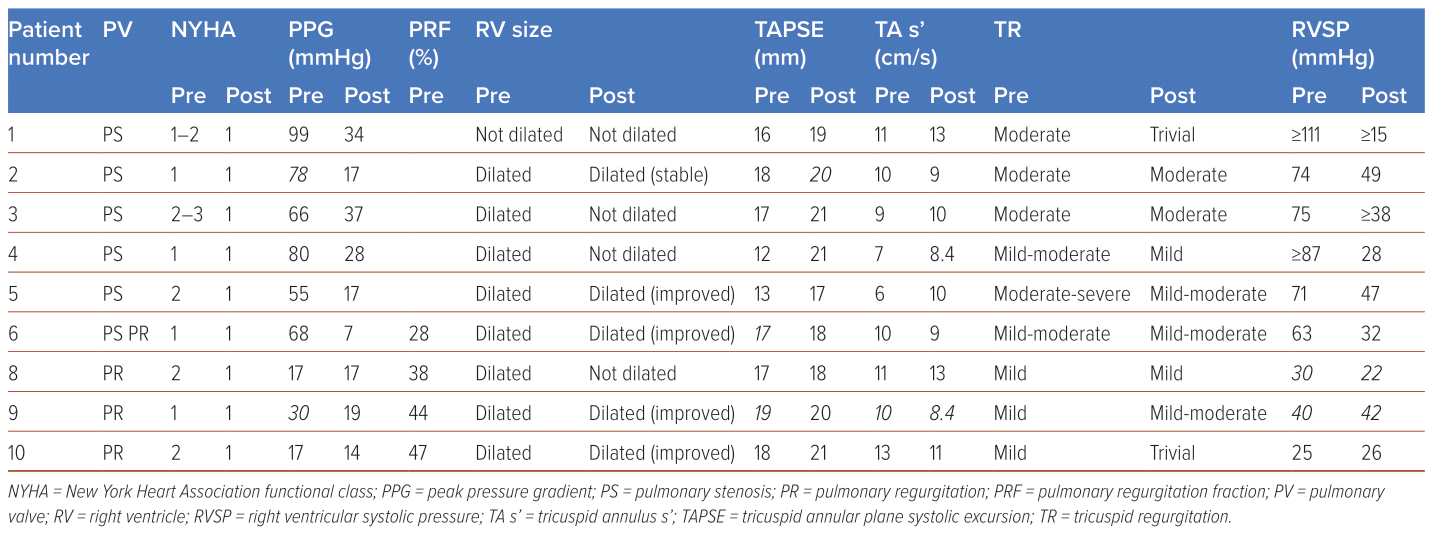
All nine patients who underwent PPVI had significant improvements in their underlying RVOT pathology (Table 2). Patients who presented with RVOT stenosis had significant improvements in PS severity. Vmax decreased from 4.25 ± 0.44 cm/s to 2.51 ± 0.38 cm/s, PPG decreased from 72.4 ± 15.6 mmHg to 26.5 ± 7.63 mmHg and MPG decreased from 47 ± 14.41 mmHg to 16.2 ± 4.45 mmHg.
Similarly, patients who presented with PR had significant improvements in PR severity. Three out of the four patients with initial RV systolic dysfunction had normalisation of RV function, with overall mean improvement in TAPSE from 16.13 ± 2.98 mm to 17.9 ± 2.95 mm, and overall mean improvement in TA S’ from 9.11 ± 1.91 to 9.72 ± 3.12. Additionally, there were significant improvements in tricuspid regurgitation (TR) and pulmonary pressures.
Two patients experienced complications of bleeding and stent fracture, both of whom were conservatively managed and had good long-term outcomes.
Discussion
Our case series above illustrates the efficacy and feasibility of PPVI in local ACHD patients from Singapore. All those who underwent successful PPVI had a significant decrease in severity in and even complete resolution of underlying RVOT stenosis or regurgitation. Improvements in RV systolic function, TR and pulmonary pressures were also noted. The 1-year survival was 100%. This is consistent with the results of other case series performed predominantly in the US and Europe that have shown improvements in New York Heart Association functional class, RV dimensions and ejection fraction, TR severity and RVSP.5–8,11,12
These studies largely showed 90% freedom from reintervention within 1 year of PPVI and 75% freedom from reintervention within 5 years.1,5,6,15 Notably, a high residual RVOT gradient, stent recoil or compression and a lack of pre-stenting were more often associated with reintervention.
Early PPVI complications include bleeding and access site issues, RVOT conduit rupture, coronary compression, acute pulmonary oedema and arrhythmias, sepsis and venous thromboembolism.1,5,6,11,15 RVOT rupture occurs in up to 9% of cases during balloon dilatation, especially in heavily calcified RVOTs and homograft conduit substrate.16 These can largely be treated successfully with covered stents without significant haemodynamic instability and only rarely require surgical intervention.17 Later PPVI complications include stent fracture, paravalvular leaks and IE.18,19 IE occurs in approximately 2–3% of cases, and may be complicated by valve explantation, reintervention or septic shock and mortality.18,19
Coronary Compression
Patient #7 was a 31-year-old Chinese woman born with pulmonary atresia with ventricular septal defect (PAVSD), who had undergone a right Blalock-Taussig (BT) shunt implantation in 1992 and a left BT shunt implantation in 1994, a Rastelli procedure with a Hancock valve in 1995 and a redo Rastelli procedure with a 20 mm aortic homograft on 14 December 2007.
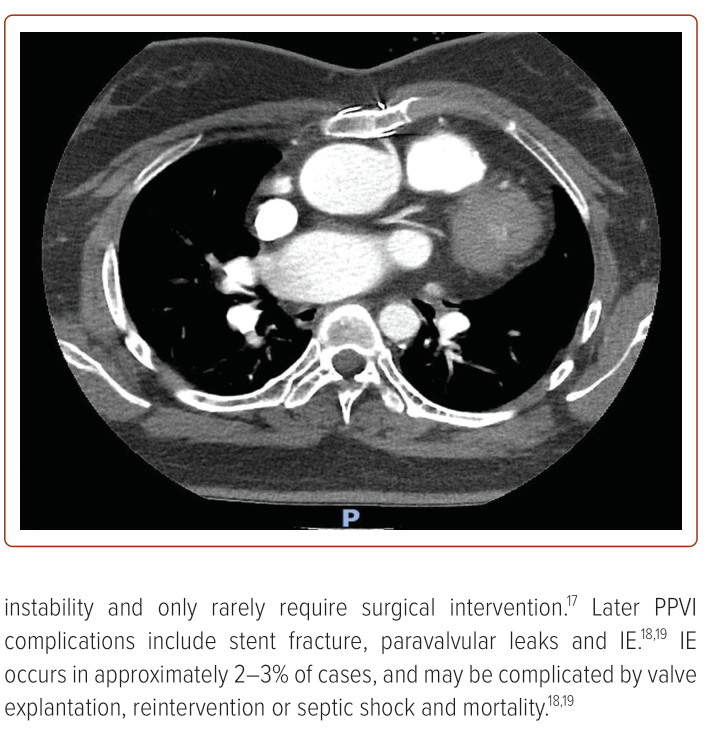
She developed severe PS (Vmax 400 cm/s; PPG 62 mmHg; MPG 37 mmHg) with moderate PR (pulmonary regurgitation fraction (PRF) = 27%; RV end-diastolic volume index (RVEDVi) 104 ml/m2; RVEF 42%). After clinical and multimodality imaging assessment showed the left anterior descending artery (LAD) coursing 6 mm inferior to the Rastelli conduit (Figure 1), it was planned that she would have a 22 mm Melody PPVI on 29 August 2022. However, pre-dilatation with a 22 mm × 45 mm True (BD) balloon was noted to cause mid-LAD compression with anterior ST elevation (Figure 2), so the PPVI was aborted. She remained stable, and repeat TTE showed an improvement in PS, with Vmax decreasing from 400 cm/s to 260 cm/s, and MPG decreasing from 37 mmHg to 18 mmHg. Subsequently, a redo Rastelli procedure with a 23 mm Perimount Magna Ease valve (Edwards Lifesciences) was performed on 6 October 2022 in view of ventricular arrhythmias, followed by subcutaneous ICD implantation on 13 October 2022.
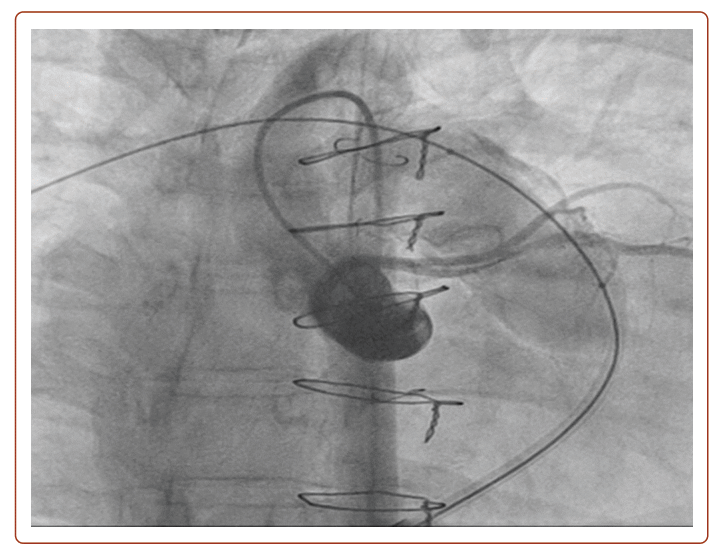
This case highlights the 5% risk of coronary compression, which is an absolute contraindication to PPVI. This is especially apparent in patients with concomitant coronary anomalies, particularly those with underlying tetralogy of Fallot (TOF) or transposition of the great arteries.20 As such, routine balloon pre-dilatation with simultaneous aortogram or coronary angiography is practised in our centre, and is essential to assess for any significant decrease in coronary flow, which suggests a risk of coronary compression. If there is any suggestion of coronary compression risk, PPVI should not be carried out as in our patient above. Should patients present with acute coronary syndrome or symptoms and signs of myocardial ischaemia after PPVI, coronary compression should be suspected and treated with emergent revascularisation comprising either percutaneous coronary intervention or surgery including percutaneously implanted pulmonary valve removal and surgical pulmonary valve replacement (PVR), if necessary.17,21,22
Access Site and Bleeding Complications
Patient #5 was a 22-year-old Chinese woman born with PAVSD and patent ductus arteriosus (PDA), who had undergone a left BT shunt in 2000 aged 6 months, an aortic homograft with ventricular septal defect patch closure and PDA ligation in 2003 aged 3 years, followed by a 22 mm Rastelli procedure with a Hancock valve and right pulmonary artery dilatation in 2006 aged 6 years.
She developed moderate to severe PS (Vmax 372 cm/s; PPG 55 mmHg; MPG 26 mmHg), mild PR, and RV dilation, hypertrophy and systolic dysfunction (TAPSE 13 mm; TA S’ 5.59 cm/s). She underwent a Melody PPVI on 30 August 2022, which also included pre-stenting with an ev3 Intrastent Mega LD (Medtronic) 36 mm × 12 mm, and post-dilatation with a Tyshak (NuMED) 22mm × 40 mm. Vmax decreased from 372 to 295 cm/s, PPG decreased from 55 mmHg to 17 mmHg, and MPG decreased from 26 mmHg to 9 mmHg, with normalisation of RV systolic function (TAPSE 17 mm; TA S’ 10 cm/s).
The patient developed retroperitoneal and pelvic extraperitoneal haematomas, which were managed conservatively based on the advice of general surgery specialists and treated with packed cell transfusions. There was no active bleeding or extension of the haematomas on serial CT abdomen scans, and her haemoglobin levels remained stable.
This case exemplifies the risk of access site and bleeding complications with PPVI in view of the large sheaths required, especially in patients of smaller builds and with smaller vasculature, as in the case of our patient who weighted 28.9 kg and had a BMI of 13.23
The Melody valve requires a 22 French gauge sheath delivery system, but has a sleeve over the sheath to aid haemostasis at the insertion site, while the Edwards Sapien valve requires either a 22 or 24 Fr gauge sheath delivery system.1 Measures such as ultrasound-guided vascular access and device closure may decrease the risk of such complications, and are routinely practised in our centre. Newer lower-profile devices that require smaller sheaths are being developed and may help to reduce the risk of these complications further.1
Stent Embolisation
Patient #10 was a 61-year-old Chinese man, with a previous TOF repair in 1969 aged 9 years. He developed severe PR due to a dehisced flail valve leaflet (PRF 54%; RVEDVi 456; RV end-systolic volume 259; RVEF 43%), and underwent a 22 mm PVR homograft in 2005. Subsequently, he developed recurrent severe PR (PRF 47%; pressure half-time 190 ms; PR index 0.75) due to a posterior cusp perforation.
An initial 22 mm Melody PPVI planned on 23 February 2022 was cancelled in view of stent embolisation during pre-stenting. Routine balloon sizing of the pulmonary annulus was initially performed with a 22 mm True balloon dilatation, with no waisting noted. However, repeat balloon inflation with a simultaneous RVOT angiogram showed no contrast into the main PA.
A decision was therefore made to pre-stent the pulmonary annulus with an uncovered CP stent (BVM Medical) of 22 mm × 40 mm. Embolisation of this stent occurred. After multiple attempts to reposition it at the pulmonary annulus were unsuccessful, the stent was post-dilatated in the left pulmonary artery with a 28 mm Tyshak NuMED balloon and a 25 mm waist. The RVOT size was remeasured at 25 mm.
After discussion and shared decision-making with the patient and members of the heart team, the patient underwent successful PPVI with a larger 29 mm Edwards Sapien S3 valve on 30 August 2022; PR improved from severe to mild, and both RV and PA pressures reduced. Notably, there was also improvement in left ventricular ejection fraction from 48% to 55.5%.
This case shows the risk of stent embolisation in PPVI, and emphasises the importance of multimodality imaging to confirm RVOT and device sizing before the procedure, including TTE, CT scans, cardiac MRI and coronary angiography.7
Stent Fracture
Patient #1 was a 25-year-old Chinese man, with a background of TOF repair in 1993 aged 3 years, who developed severe PR with an RVOT aneurysm. He underwent a 25 mm pulmonary homograft in 2013, and a subsequent redo 25 mm pulmonary homograft in 2016 for prosthetic Granulicatella IE complicated by leaflet perforations and septic emboli to the lungs. Subsequently, he developed severe supravalvular and valvular PS (Vmax 499 cm/s; PPG 99 mmHg; MPG 61 mmHg). Percutaneous balloon pulmonary valvuloplasty was attempted with a Numed 18 × 40 mm balloon on 24 January 2017; however, there was a significant residual pullback gradient of 80 mmHg despite three balloon dilatations.
The patient underwent a 22 mm Melody PPVI on 23 June 2017, including pre-stenting with a CP 22 × 39 mm and post-dilation with an Atlas (Stryker) 22 × 40 mm. There was resolution of PS, with a decrease in pullback gradient from 80 mmHg to 23 mmHg (in view of residual RV mid-chamber band), Vmax from 499 cm/s to 290 cm/s, PPG from 99 mmHg to 34 mmHg and MPG from 61 mmHg to 16 mmHg.
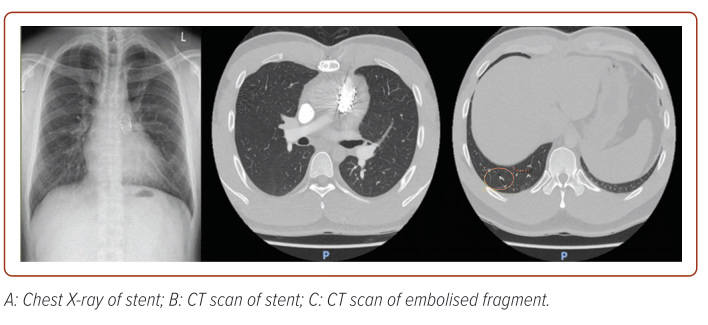
On serial follow-up in 2019, one stent strut was noted to be fractured in 2019; this progressed to three stent struts fractured in 2021, complicated by embolisation of a small stent fragment to the right lower lobe lateral sub-segmental artery (Figure 3). The patient remained well, asymptomatic and stable, with subsequent stable imaging findings (Vmax 300 cm/s; PPG 40 mmHg; MPG 22 mmHg; on latest TTE on 27 July 2023), and remains on serial follow-up with our centre.
Stent fracture occurs in up to 30% of PPVI cases, and is associated with a younger age, a high RVOT gradient, implantation into the native RVOT, smaller conduits, a lack of calcification, stent recoil or compression with eccentricity index >1.1, and valve apposition to the anterior chest wall.24–26
Stent fractures are classified as type 1 (no loss of stent integrity), type 2 (loss of stent integrity), and type 3 (separation or embolisation of the fractured segment) under the Nordmeyer classification system.26 Type 1 stent fractures are the most common, are identified on serial radiography or fluoroscopy and are treated conservatively. Type 2 and 3 fractures may be treated with valve-in-valve procedures; type 3 stent fractures may require surgery for distal embolisation.26 Pre-stenting before Melody valve implantation should be done to decrease the risk of stent fracture, and is carried out routinely in our centre.15,24,27
Conclusion
We present our early local experience with PPVI in ACHD patients at a single local tertiary centre. PPVI has proven to be a safe and efficacious procedure within our case series and has the potential to significantly improve PS and PR, RV and LV systolic function, as well as pulmonary pressures. However, precautions must be taken to decrease the risk of complications such as coronary compression, access site bleeding, device embolisation and stent fracture. 
Clinical Perspective
- Percutaneous pulmonary valve implantation is being increasingly used to treat right ventricular outflow tract dysfunction in adults with congenital heart disease.
- Percutaneous pulmonary valve implantation is a safe and efficacious procedure and has the potential to significantly improve pulmonary stenosis and pulmonary regurgitation, associated symptoms of pulmonary stenosis and regurgitation, right and left ventricular function, and pulmonary pressures.
- Precautions must be taken to minimise the risk of complications such as coronary compression, access site and bleeding complications, device embolisation and stent fracture.