Pathological deposition of calcium in the intimal and medial layers of the arterial wall is associated with old age, chronic kidney disease and diabetes among conventional cardiovascular risk factors, and represents a therapeutic challenge for interventional cardiologists worldwide.1
Patients with coronary artery calcification (CAC) are less likely to have undergone complete revascularisation, and the presence of CAC increases the risk of major adverse cardiac events (MACE) and mortality after percutaneous coronary intervention (PCI).2,3 CAC can result in stent underexpansion, leading to stent thrombosis and in-stent restenosis (ISR).4,5 Placing drug-eluting stents in calcified lesions has been shown to result in disruption of the polymer, leading to non-uniform drug delivery and, consequently, a higher risk of in-stent restenosis.6
Various interventions have evolved for pre-treating lesions with the goal of modifying calcified plaque prior to stent implantation to mitigate risks of stent under-expansion or malapposition. These include balloon-based techniques where non-compliant or cutting/scoring balloons are dilatated to very high pressures in an attempt to break calcifications, as well as ablation-based techniques, such as rotational atherectomy (RA), excimer laser atherectomy and orbital atherectomy, which aim to debulk or crack calcifications while soft tissue flexes away.7 More recently, intravascular lithotripsy (IVL) has provided a valuable alternative for treating deeply embedded concentric calcium by inducing fractures using ultrasonic pressure waves.3
Combination therapy involving two or more of the aforementioned methods is occasionally required for severely calcified lesions. One contemporary example is RotaTripsy, which relies on RA to debulk intimal calcium to facilitate the delivery of stents or balloons, accompanied by IVL to fragment circumferential deep calcium plaques.8 Calcium modification after RA provides a round, concave and polished calcium surface, while calcium modification after IVL results in complete discontinuity of calcium, which indicates calcium fracture. Often, both of these need to be carried out to get a good platform for stent implantation. Furthermore, IVL is an alternative to repeating RA runs with larger burrs, which increases the risk of angiographic complications and may also require the use of larger access sheaths with the associated increase in risk of access site complications.9
Despite this strategy gaining in popularity, data and outcomes on patients treated with RotaTripsy are scarce, being confined to small case series with limited follow-up. We present data from a cohort of patients treated with RotaTripsy over 2020–2022 at our tertiary centre.
Methods
Ethical Statement
The design of this registry has been described in prior publications by our group.10 In summary, following the introduction of IVL, local regulatory directives mandated the establishment of a registry for the purposes of quality improvement audits. All IVL cases from our institution were included in this registry, with data anonymised at the point of collection. The need to gain informed consent for the subsequent collection and use of this anonymised data was waived with permission from our central institutional review board.
Study Design
This was a single-centre, retrospective study conducted at a high-volume tertiary centre in Singapore. PCI cases between May 2020 and December 2022 were identified in which both RA and IVL were employed in the same procedure.
Baseline demographics and clinical outcomes data were collected from the Singapore Cardiac Data Bank, which was established in 2000 as a national data bank of cardiovascular diseases and procedures, and contains epidemiological, clinical and procedural data. It is frequently used for national audit, quality improvement and research purposes.
Participants
As per local regulations, participants were eligible for IVL therapy only if they had either: severe calcification on intravascular imaging as defined as ≥270° arc of calcium; or angiographic evidence of severe calcification that devices were unable to cross or a non-compliant balloon was unable to expand adequately.
Participants received RA as per standard indications for heavily calcified lesions if this was deemed suitable by the primary operator. All received both RA and IVL within the same index procedure. Cases where values in age, sex or complications were missing were excluded from the study. Four patients had more than one RotaTripsy procedure during the study period. In these patients, only the first procedure was included.
Study Device and Procedures
IVL was performed using a Shockwave IVL catheter (Shockwave C2; Shockwave Medical) and RA performed using a Rotablator (Boston Scientific). Methods of deploying IVL and RA equipment have been described in previous reports published by our group.10
A planned combined RA and IVL approach was identified as: explicit documentation by the operator in the procedure report; or implied from the use of IVL before RA, or directly after RA without a trial of any other balloon-based therapy (non-compliant/scoring/cutting) in between.
All patients received dual antiplatelet therapy prior to PCI, namely aspirin with either clopidogrel or ticagrelor, and intraprocedural heparin in accordance to established clinical guidelines.11,12
Endpoints
The primary endpoints were in-hospital and 30-day MACE, defined as a composite of all-cause mortality, MI, target vessel revascularisation (TVR) or stroke. The secondary endpoints were procedural success, in-stent thrombosis, intraprocedural perforation or persistent slow or no-reflow.
In-hospital MI included both peri- and post-procedural MI. Periprocedural MI was defined as peak post-PCI creatine kinase-myocardial band levels >3 × the upper limit of normal with or without new pathological Q waves, as per the ORBIT-2 study.13 Otherwise, MI was defined as per the fourth universal definition of MI.14 Procedural success was defined as per the criteria laid out in the DISRUPT CAD III study, which entailed successful stent delivery with <30% residual angiographic stenosis and without in-hospital MACE.3
Statistical Analysis
Continuous data was given as median (interquartile range). Categorical data is presented as counts and proportions (%). Pearson’s χ2 test and Fisher’s exact test were performed to compare groups regarding categorical variables, and the Mann-Whitney U-test was performed for continuous variables. A p value of ≤0.05 was considered statistically significant. Logistic regression was performed to predict risk factors for outcomes of in-hospital MACE. Statistical analysis was performed using R software version 4.1.2 (R Foundation).
Results
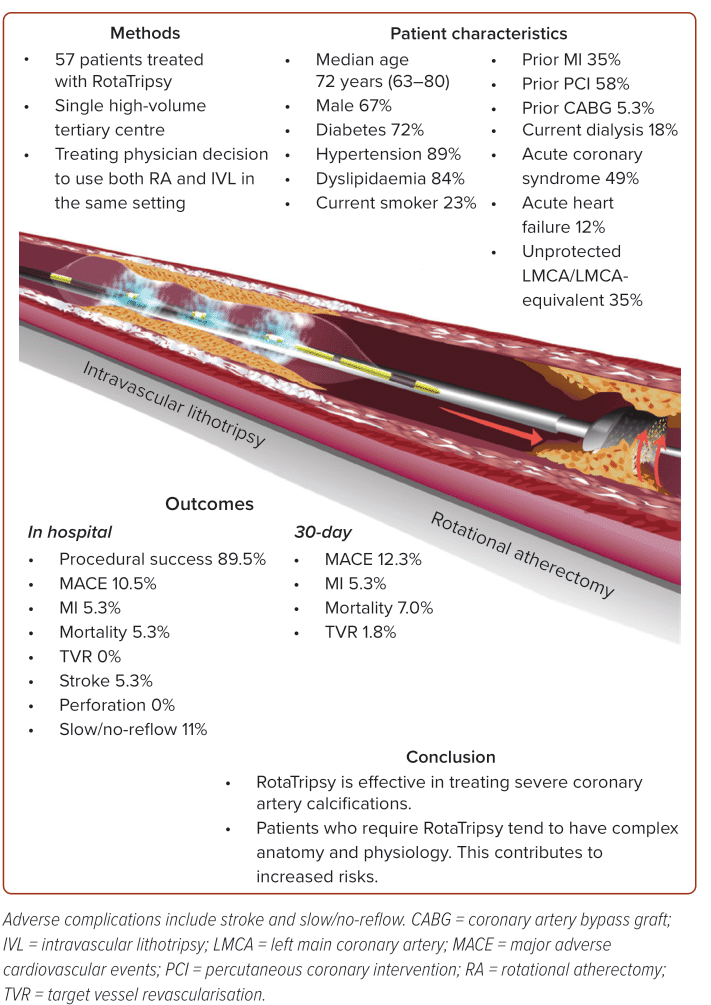
Between May 2020 and December 2022, a total of 57 patients received PCI in which both RA and IVL were carried out. Twenty-three (40%) of cases were conducted in the context of acute coronary syndrome (ACS), including three cases (5.3%) of ST-elevation MI. The majority of the cases (75%) had triple vessel involvement, and 25% involved repeat PCI for in-stent restenosis.
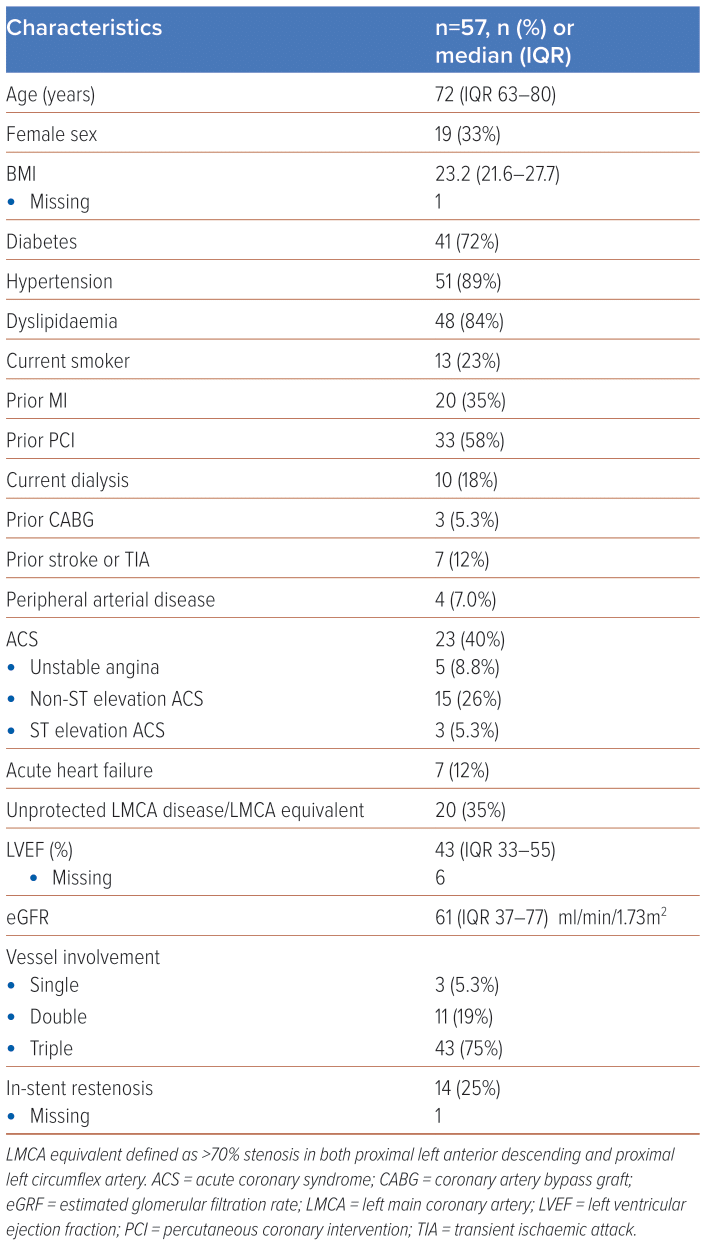
Baseline patient characteristics are shown in Table 1. Median age was 72 years (IQR 63–80) and 33% of patients were women. There was a high prevalence of cardiovascular comorbidities, including diabetes (72%), hypertension (89%) and dyslipidaemia (84%). Of note, 18% of patients were on dialysis and 5.3% had prior coronary artery bypass graft surgery. Thirty-five per cent of patients had prior MI and 58% had prior PCI. The median left ventricular ejection fraction was 43% (IQR 33–55%).
In the vast majority of cases (95%), RA was used before IVL. IVL was also mostly used (in 67% of cases) as a bailout strategy after RA failed to achieve sufficient calcium modification, frequently evidenced by a residual waist visible during non-compliant balloon dilatation despite initial RA therapy. In the rest of the cases, an upfront decision had been made by the proceduralist to employ a dual RA and IVL strategy for plaque modification.
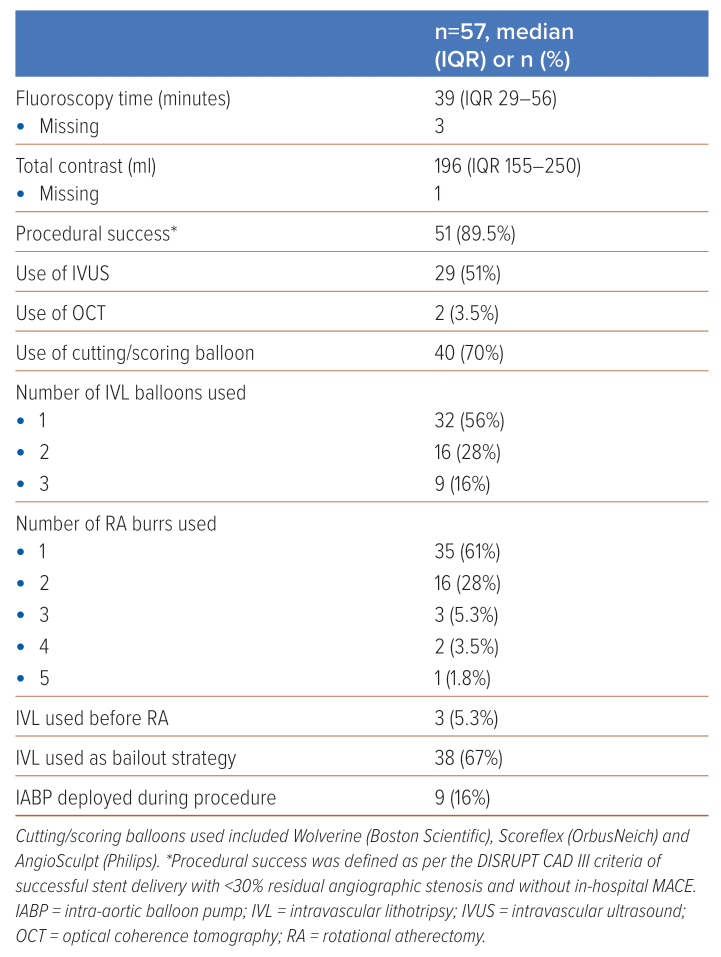
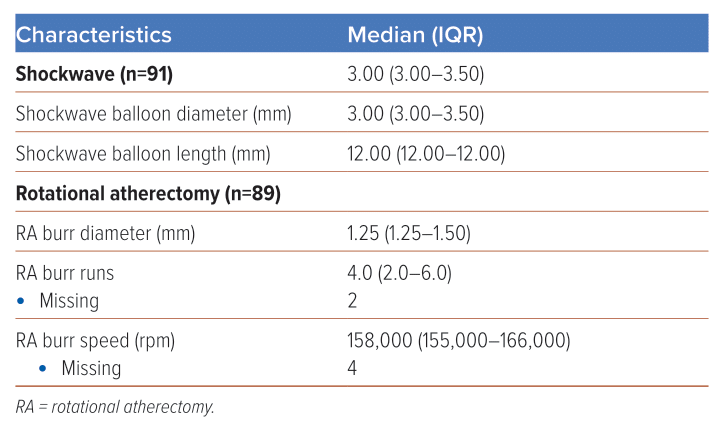
Median fluoroscopy time for these RotaTripsy cases was 39 minutes (IQR 29–56) and median contrast volume was 196 ml (IQR 155–250). Seventy per cent of cases concomitantly involved cutting balloons (e.g. Wolverine [Boston Scientific], AngioSculpt [Phillips] or Scoreflex [OrbusNeich]) and, in approximately half of all cases, adjunctive imaging modalities such as intravascular ultrasound or optical coherence tomography were employed. In 44% of cases, more than one Shockwave balloon was used and, in 39% of cases, more than one burr was used, with a median of four runs per burr and a median burr speed of 158,000 rpm. Full procedural details are documented in Table 2. Table 3 provides further details of the RA and IVL devices used.
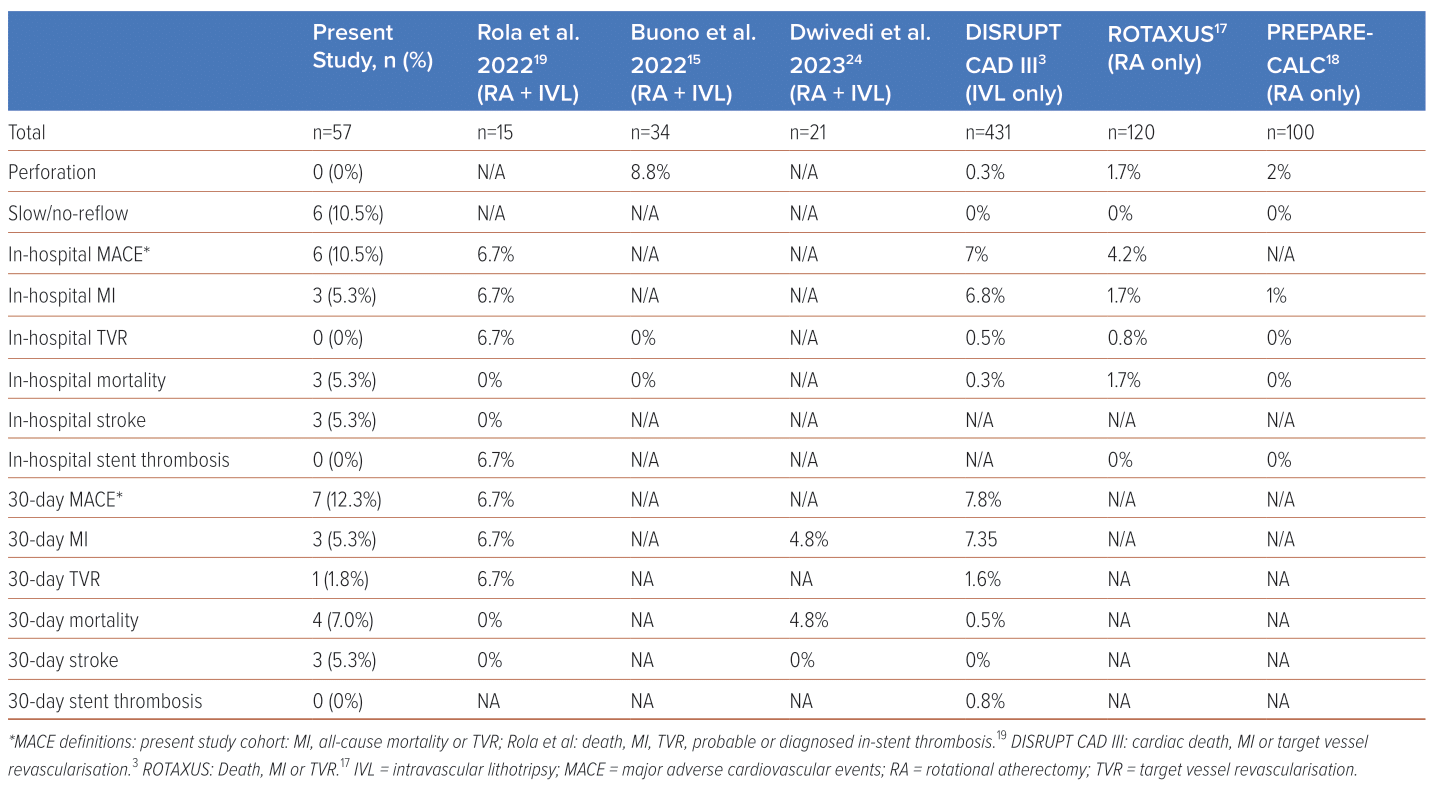
Most (89.5%) cases achieved procedural success. A relatively high proportion (10.5%) were complicated by slow/no-reflow. Additionally, there were three in-hospital deaths (5.3%) and three cases of in-hospital MI (5.3%), giving an overall in-hospital MACE of 10.5%. There were no cases of coronary perforation or in-stent thrombosis. There were three cases of stroke (5.3%). At 30-day follow-up, there was one additional death and one additional case of target vessel revascularisation, yielding a 30-day MACE of 12.3%. Patient characteristics and outcomes are summarised in Figure 1 and a full comparison of in-hospital, and 30-day outcomes can be found in Table 4.
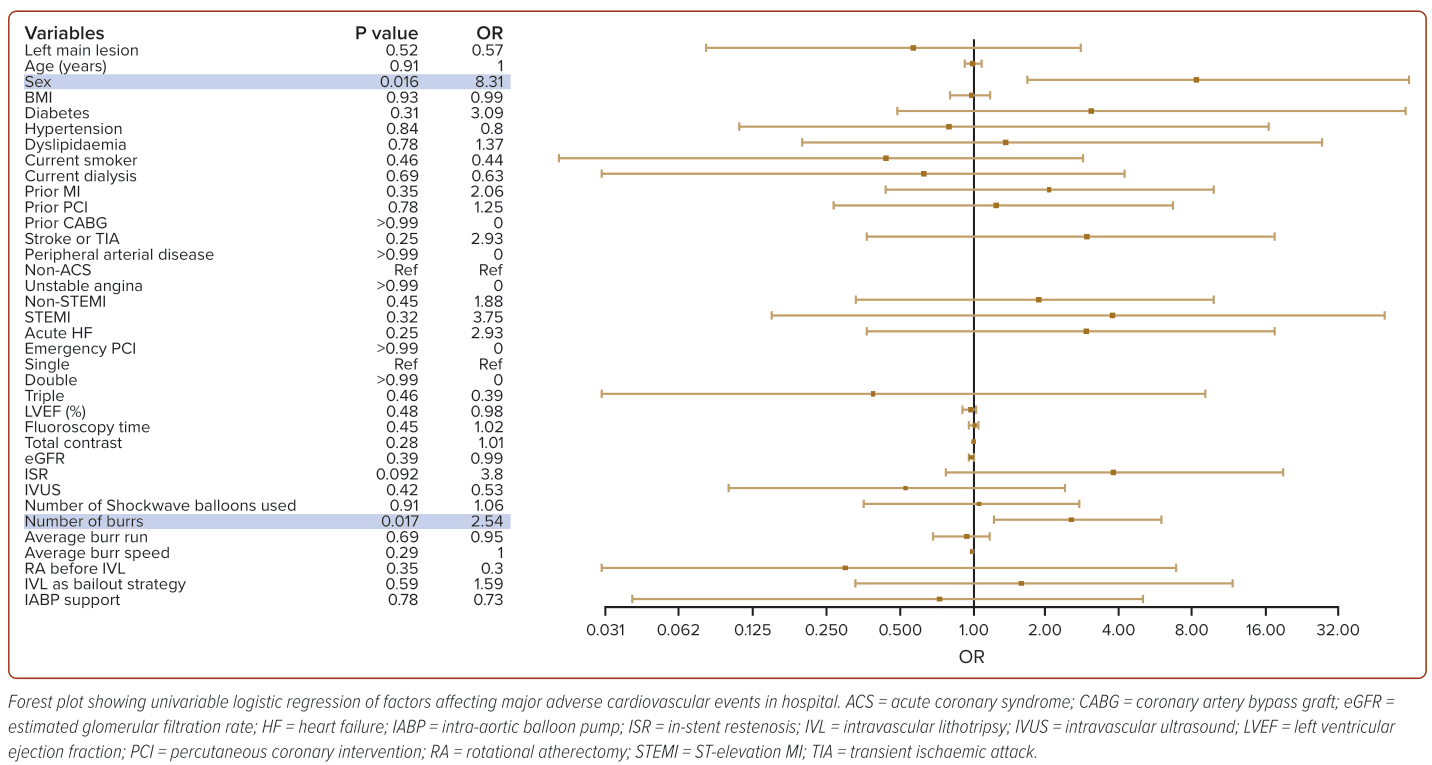
To delineate the factors contributing to MACE, we performed univariable logistic regression (Figure 2), which revealed that in-hospital MACE was most significantly predicted by female sex (OR 13.2) and the number of RA burrs used (OR 2.57).
Discussion
Heavily calcified plaque remains a significant challenge for interventional cardiologists. While RA and IVL have been compared against each other as alternative calcium-modification strategies and shown to have comparable rates of efficacy and in-hospital outcomes, theoretically they should be complementary, with RA targeting luminal calcification for debulking and facilitating the delivery of IVL devices that may assist in fracturing concentric calcium rings that extend deeper into the vessel wall.10,15,16 Our study validates the use of a combined RA and IVL strategy as an efficacious procedure with high procedural success.
Despite the growing popularity of this approach, data on patient outcomes with RotaTripsy remains sparse, being confined to case series and a few registries with small numbers of patients. To the best of our knowledge, our study includes the largest registry so far of patients treated with a combined RA and IVL approach.
Additionally, our study provides insight into a context in which RotaTripsy was applied at the discretion of the primary operator as long as patients fulfilled the criteria for RA or IVL as per their individual indications with no specific exclusion criteria. It included a high proportion of patients with ACS (40%), acute heart failure (12%) and unprotected left main coronary artery (LMCA)/LMCA-equivalent lesions (35%); such patients had been excluded from the ROTAXUS, PREPARE-CALC and DISRUPT CAD III cohorts.3,17,18 RotaTripsy was used to treat ISR in 23% of our patients, whereas ISR was excluded from the ROTAXUS and PREPARE-CALC studies.17,18 The present study thus adds to the growing body of evidence that a combined RA and IVL strategy may be used in the acute setting and in a sicker group of patients with heavily calcified lesions.
The in-hospital MI rate of 5.3% in our registry was grossly comparable to the 6.8% rate in DISRUPT CAD III, and in line with other RotaTripsy registries, but considerably higher than the RA cohorts (1.7% in ROTAXUS and 1% in PREPARE-CALC).3,17,18,19 All in-hospital MI cases were detected immediately after the procedure and were deemed to be related to PCI.
Mortality was also higher in our cohort (5.3%) than in the RA-only or IVL-only studies. A review of the three in-hospital deaths revealed two cases of cardiac mortality, with the final patient deteriorating following a massive cerebrovascular infarct. No in-hospital mortalities were reported in the other RotaTripsy registries, despite them also recruiting a sizeable proportion of patients with ACS; however, care must be taken in drawing definitive comparisons from small sample sizes.15,19
The high rates of MACE, MI and mortality may be explained by the fact that the strict requirements for combined RA and IVL select for a subset of patients with significant CAC. Patients with a high burden of CAC tend to have complex disease both anatomically and physiologically, which predisposes them to worse outcomes.20
Furthermore, the comparatively higher rates of adverse outcomes in our cohort as compared to the RA-only or IVL-only trial-based cohorts likely relate to its nature; our cohort captures arguably more complex patients.
Apart from our study not excluding patients with ACS, acute heart failure and LMCA/LMCA-equivalent lesions as mentioned above, patients in our cohort had a lower median ejection fraction of 44% compared to 55% in the other studies, and a considerable proportion (16%) required intraprocedural intra-aortic balloon pump support.17,18
In addition, 18% of patients in our cohort were on dialysis, whereas patients on dialysis were excluded from the DISRUPT CAD III IVL cohort and only 6.7% of patients in the ROTAXUS cohort had chronic renal failure.3,17 Such dialysis patients are known to have dysregulation of mineral metabolism, with a greater CAC score and correspondingly higher MI risk.21
The relatively large number of patients in our cohort with intra-procedural slow/no-reflow (11%) is likely to be a result of the high calcium burden with increased risk of distal embolisation, which highlights this as an significant complication. Of the six patients with this complication, two had in-hospital MI while another died in hospital. However, our cohort was underpowered to draw any further conclusions on the relationship between the slow/no-reflow phenomenon and MACE.
Three patients (5.3%) from our cohort experienced in-hospital, procedure-related cerebrovascular events, suggesting that this is a complication that clinicians should be mindful of, despite it not being an endpoint recorded in comparator studies. There were no cases of coronary perforation; in comparison, the ROTAXUS and PREPARE-CALC studies that employed an RA-only strategy reported 1.7% and 2% perforation rates, respectively.
The use of a higher numbers of burrs and female sex were associated with in-hospital MACE. The use of more burrs likely suggests more complex disease and hence understandably poorer outcomes. The observation that female sex is associated with poorer outcomes reflects similar observational findings by the Coronary Artery Calcium Consortium that detectable CAC was associated with 1.3-fold higher hazard for cardiovascular death among women compared with men, and that cardiovascular mortality was higher among women with more extensive, numerous or larger CAC lesions.22
In our logistic regression analysis, the odds ratio for ST-elevation ACS, acute heart failure and ISR were numerically high, despite not achieving a significant p value. These may be potential risk factors predisposing people to MACE, although the data did not reach statistical significance due to the relatively small number of patients in our study. These risk factors are an area for potential future research involving registries with more patients.
Limitations
Our study is limited as it is based on non-randomised retrospective data collected from a small number of patients at a single centre. Furthermore, the study period coincided with that of initial local experience with IVL, where operators were still gaining exposure to the device. Comparisons with other RotaTripsy registries are limited by small sample sizes and differences in baseline patient presentations, especially regarding the proportion of patients with ACS.
Additionally, the use of intravascular imaging was limited, with roughly half of the patients undergoing RotaTripsy without prior intravascular imaging. The use of intravascular imaging is not reimbursed in our country, so making use of these adjuncts during PCI is not routine practice. Nevertheless, the authors appreciate that intravascular imaging will improve the assessment of coronary calcification and greatly assist operators in planning their approach to calcium modification and assessing the results of their interventions, as suggested by the recently reported RENOVATE-COMPLEX-PCI study.23 There was some suggestion from our regression analysis that the use of intravascular ultrasound was associated with lower MACE in our study, although not significantly so (OR 0.44; 95% CI [0.06–2.49]; p=0.37), which lends support to the use of intravascular imaging during these complex interventions.
It was also not possible to identify for certain whether the slow/no-reflow complications were related to RA alone as these procedures were often complicated, involving many burr runs; in addition, in at least half of the cases, slow/no-reflow was observed towards the end of the procedure after both RA and IVL had been performed.
Conclusion
This study presents a cohort of 57 patients in whom a combined RA and IVL strategy, RotaTripsy, was used for calcium modification during lesion preparation, as per the discretion of the primary operator. Overall, while RotaTripsy was effective in facilitating stent delivery and restoring Thrombolysis in MI 3 flow in a real-world setting, patients who required such advanced calcium modification therapies tended to have complex anatomy and physiology, which increase the risk of this procedure with major adverse complications of stroke, slow/no-reflow, MI and death that proceduralists should be mindful of.