Left main (LM) coronary artery disease is an important high-risk coronary lesion, owing to the substantial proportion of myocardium at risk.1 Revascularisation for LM disease has progressed in the past few years. Earlier, medical therapy alone had a 3-year survival of 40–60%.2 Then, studies, such as the Coronary Artery Surgical Study and European Coronary Surgery Study group, showed the mortality benefit of coronary artery bypass grafting (CABG) over medical therapy in patients with significant LM disease (defined as >50% stenosis).3,4
More recent trials, including the SYNTAX, EXCEL and NOBLE studies, have compared percutaneous coronary intervention with CABG for LM disease, and have shown promising results.5–11 Consequently, percutaneous coronary intervention (PCI) is now starting to be considered as a feasible option for revascularisation in LM. However, significant coronary artery calcification is a deterrent to successful PCI, and limits both in-hospital and long-term results.12 The calcification leads to a difficulty in delivering hardware, increased risk of dissection, perforation or improper stent deployment.12 A combined analysis of the ACUITY and HORIZONS-AMI studies demonstrated poorer stent expansion, periprocedural complications and residual stenosis in calcified vessels, translating into higher major adverse cardiac events (MACE) on follow-up.13 It is noteworthy that calcification of the LM was found in 31–43% of patients in LM intervention studies, which is higher than the 17–35% incidence of calcium reported in the general population undergoing PCI.9,10,12
As a calcified LM greatly raises the SYNTAX score and increases procedural complexity, CABG has been the conventionally preferred modality for revascularisation in such patients.14 Nonetheless, with recent advances in PCI techniques, intravascular imaging and pharmacotherapy, PCI has become an important alternative to CABG, especially for haemodynamically unstable or high-risk surgical patients.15
Rotational atherectomy (RA) was the only dedicated device for calcified lesions for the past three decades, but now, modified balloon catheters, such as cutting, scoring and high-pressure balloons, and newer modalities, such as intravascular lithotripsy (IVL), have improved procedural outcomes.12,16 Two European registries showed that RA of LM improved clinical outcomes compared with conventional therapy.17,18 One of the studies did show an increased in-hospital mortality and target vessel revascularisation in the LM-RA group when compared with the non-LM-RA group, which is consistent with the high risk attributed to LM lesions.18
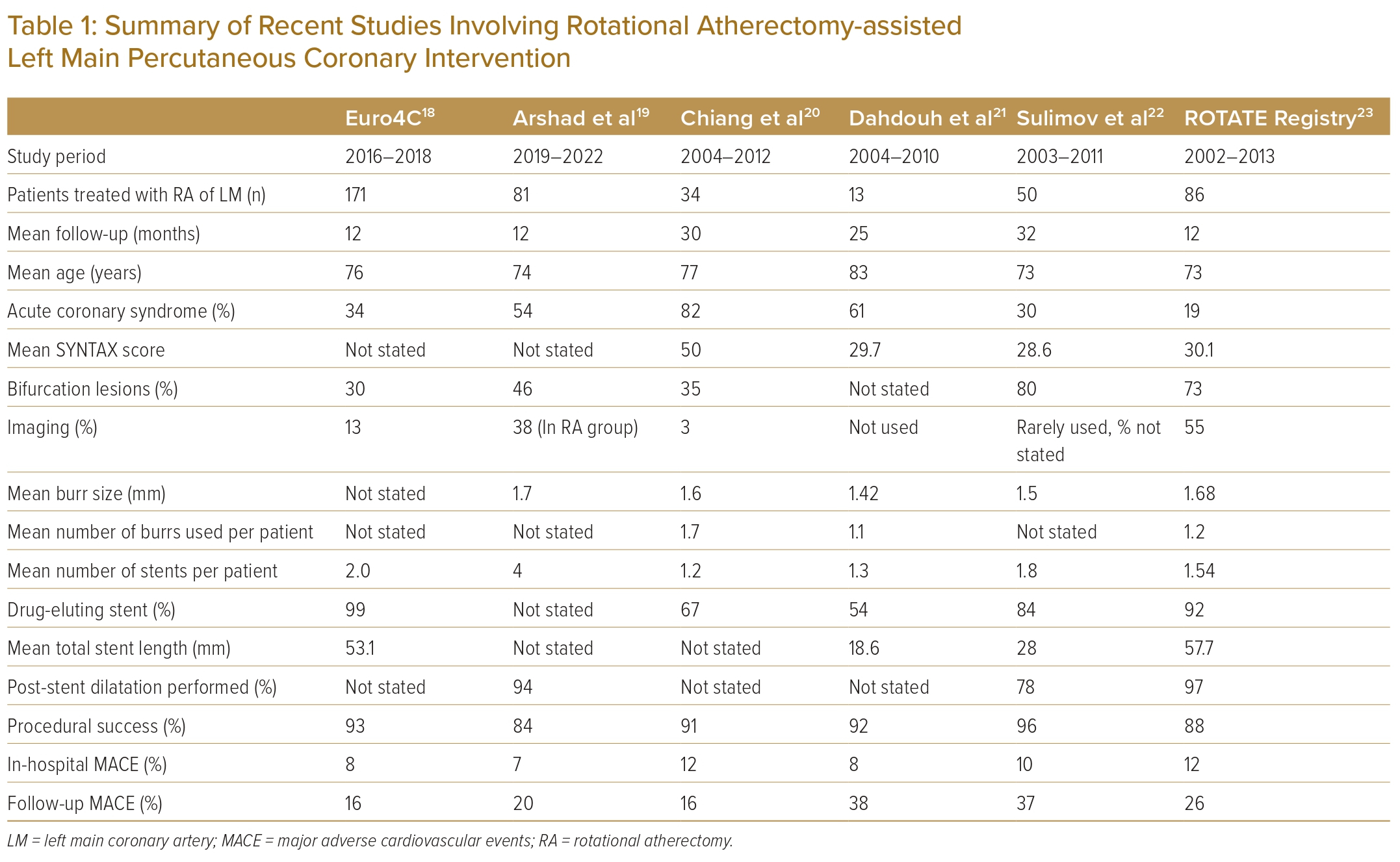
Recent studies of PCI in calcified LM lesions are summarised in Table 1.18–23 These have established the safety of RA and IVL in LM-PCI. However, data from the Indian subcontinent are lacking. It is of particular interest that surgeons performing CABG in India face unique challenges, which include late presentation, diffuse disease, smaller coronary artery size and reluctance/financial constraints of patients in undergoing major surgery.24 Therefore, assessing the feasibility and outcomes of RA-assisted PCI in a developing country, where CABG may not be easily available, is imperative.
This study provides our experience of 55 consecutive patients who underwent RA-assisted PCI for calcified LM disease, and their angiographic and clinical outcomes.
Materials and Methods
This was a retrospective study performed at a government tertiary care referral centre of North India. All consecutive patients aged ≥18 years who had undergone RA for LM coronary artery disease under the investigators from August 2018 to May 2023 were included in the study. Relevant clinical, laboratory and angiographic parameters were retrieved from the medical record database and were analysed. Follow-up of patients had been performed either in the outpatient department or by telephone.
Angiography and PCI were performed by a team experienced in RA, imaging and LM interventions. The initial calcium burden of the lesions was assessed by angiography.25 Intravascular ultrasound (IVUS) was used for most patients. Significant disease was considered as >50% stenosis for LM and >70% for the other major coronary arteries. LM disease was considered unprotected if there was no distal bypass graft to the left anterior descending (LAD) or left circumflex (LCX) coronary arteries. The SYNTAX score was calculated for all patients.
Patients and their families were appropriately counselled for CABG versus PCI by both the cardiology and the surgical teams. The decision to proceed to PCI was considered only after a thorough review of the clinical and angiographic data, cardiothoracic and vascular surgery consult, patient preference, and after obtaining a written informed consent.
PCI was performed using standard institutional protocols. All patients were given a loading dose of aspirin and a P2Y12 inhibitor (clopidogrel/ticagrelor/prasugrel). Heparin was used intraprocedurally to maintain an activated clotting time of 300–350 seconds. Intra-aortic balloon pump or Impella support was used if needed. An algorithmic approach was followed to modify calcium. For patients with moderate to severe angiographic calcification, RA was initially performed with a ≤0.5 burr:artery ratio. Following this, if subsequent intravascular imaging showed nodular calcium and favourable wire bias, we upsized the burr to a size of 1.75 or 2.0 mm. Then, if no nodular calcium was seen, the lesion was predilated with a non-compliant or cutting balloon, with size based on vessel measurements taken on IVUS, and a stent was deployed if the calcium was cracked on imaging. If calcium was not modified, we either further upsized the burr or used an IVL/Open and is a Super High pressure NC balloon (SIS Medical AG), as per imaging characteristics. A detailed flowchart of this protocol is shown in Figure 1.
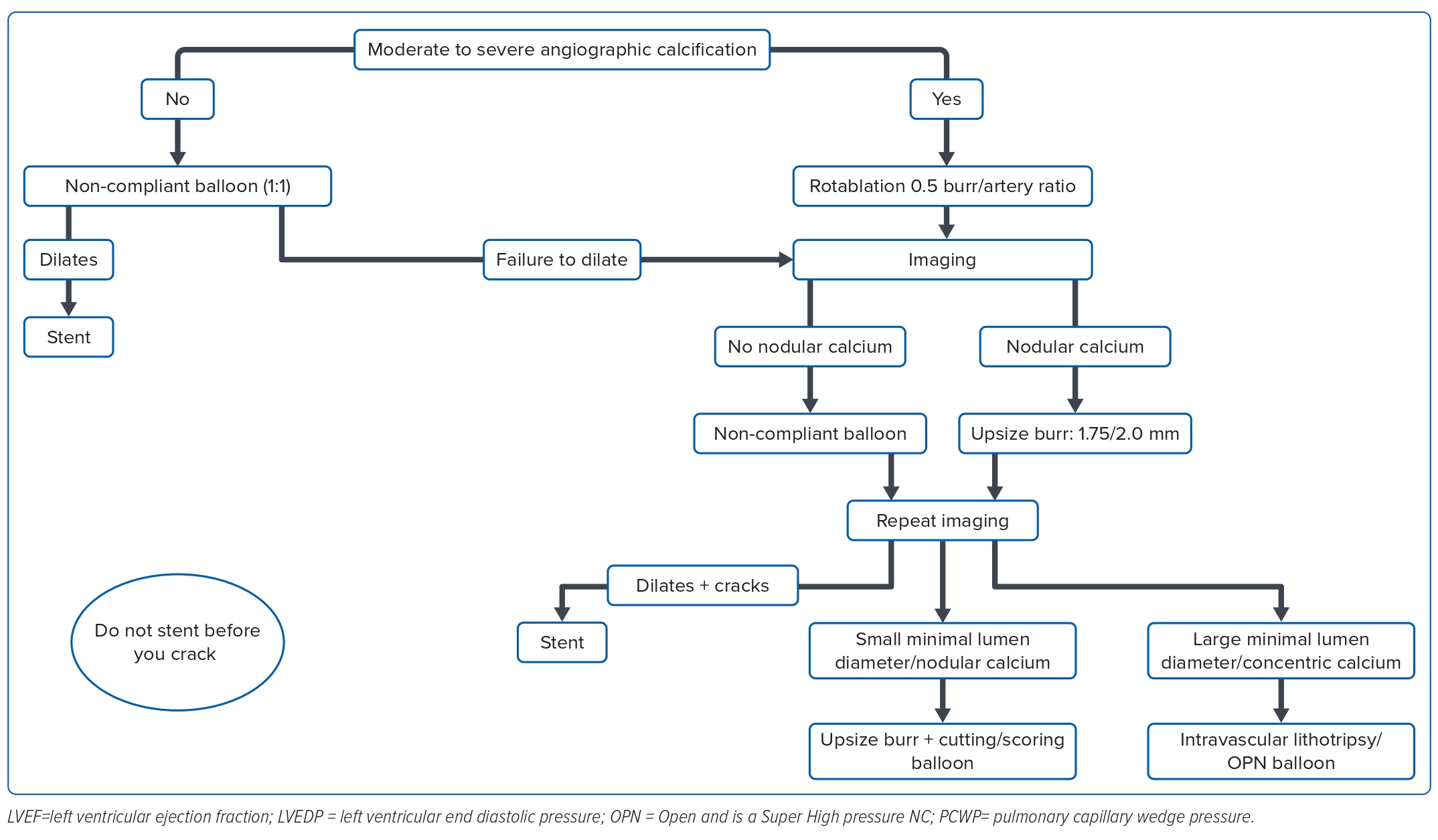
For RA, a 0.009" floppy ROTAWIRE (Boston Scientific) was advanced through the lesion using the microcatheter-assisted wire exchange technique. The ROTABLATOR RA system (Boston Scientific) was used to perform RA. The burrs were carefully advanced, at a speed of 180,000–200,000 RPM. To avoid slow flow, intracoronary nicorandil 2 mg was given before ablation, and the duration of each RA run was limited to <10 seconds with at least 30-second intervals between subsequent runs. Side-hole guiding catheters were used in patients with severe ostial LM disease. After RA, the target lesion was crossed with routine coronary wires, and further plaque modification and stenting was performed under IVUS guidance in most patients. Procedural success was defined by a thrombolysis in MI grade 3 flow and a <30% residual stenosis. The patients were closely monitored postoperatively in the cardiac care unit for at least 1 day.
During follow-up, occurrence of any MACE, including new MI, stroke, cardiac/all-cause death, target vessel revascularisation/target lesion revascularisation were noted.
Descriptive statistical analysis was performed. Demographic characteristics were summarised with descriptive statistics, including the mean and SD for continuous variables, and frequency and percentages for categorical variables.
Results
Demographic Characteristics
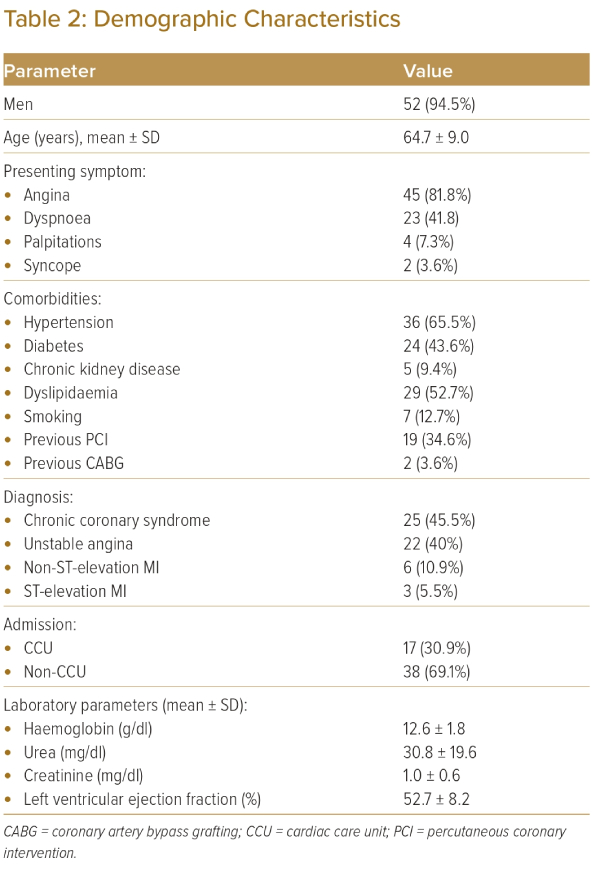
From August 2018 to April 2023, 55 consecutive patients who underwent RA-assisted LM-PCI were included in the study. The mean age was 64.7 ± 9.0 years, and 52 patients (94.5%) were men. The most common presenting symptom was angina (n=45, 81.8%), followed by exertional dyspnoea (n=23, 41.8%). Clinical presentation was as follows: 25 (45.5%) had chronic stable angina, 22 (40.0%) had unstable angina, six (10.9%) had non-ST elevation MI and three (5.5%) had ST-elevation MI. Hypertension was the most frequently associated comorbidity (n=36, 65.5%), followed by diabetes (n=24, 43.6%). A total of 19 patients had previously undergone PCI, and two had undergone CABG. A total of 17 (30.9%) patients were admitted directly into the cardiac care unit, while the rest (n=38, 69.1%) were non-cardiac care unit admissions. The mean left ventricular ejection fraction on echo was 52.7 ± 8.2%. Further details are summarised in Table 2.
Procedural Characteristics
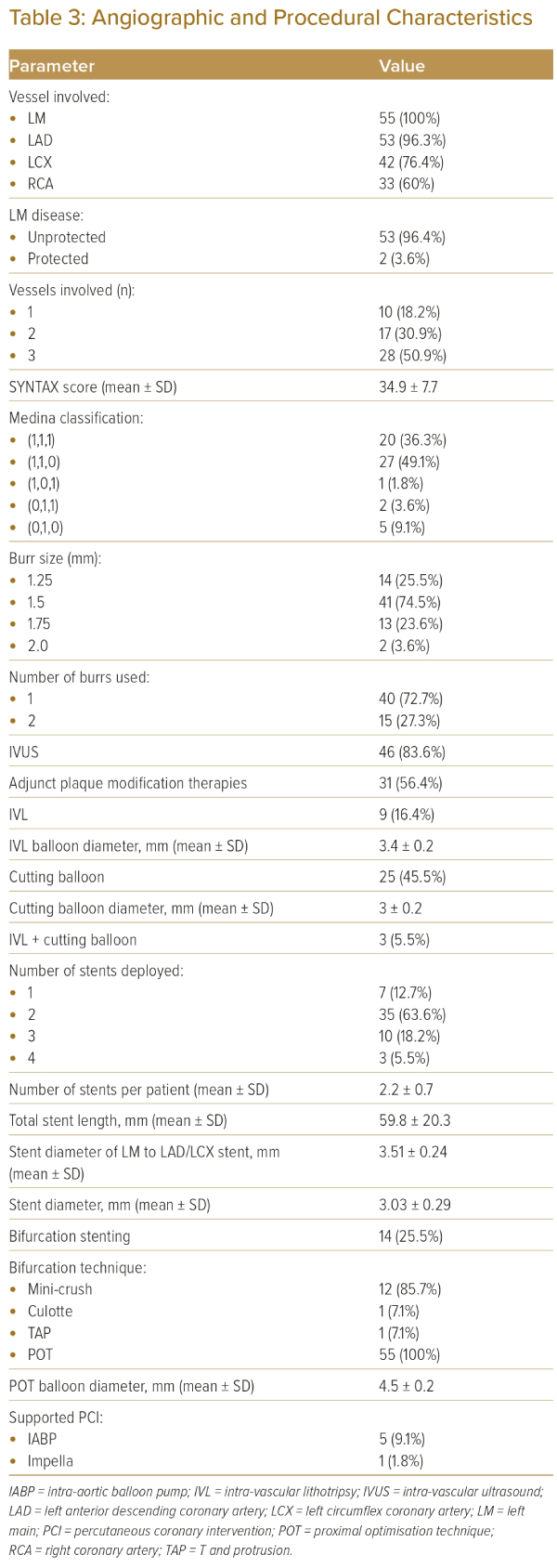
A total of 28 patients (50.9%) had triple-vessel disease. The most commonly involved vessel (along with the LM) was the LAD, which was diseased in 53 patients (96.3%). The mean SYNTAX score was 34.9 ± 7.7.
A total of 22 patients (40%) had true LM bifurcation lesion (Medina class 1,1,1 or 0,1,1). All PCIs were performed through the 7 Fr transfemoral route using 7 Fr guide catheters.
The need for RA and other plaque modification techniques was at the discretion of the primary operator, and based on the aforementioned algorithm (Figure 1). The median burr size was 1.5 mm. More than one burr was used in 15 (27.3%) patients. The mean number of burrs used per patient was 1.3. Large burrs (1.75 and 2.0 mm) were used in 15 (27.3%) patients. Simultaneous RA of both LAD and LCX was performed in 10 (18.2%) patients. Concomitant cutting balloon was used in 25 patients (45.5%), and IVL was performed in nine (16.4%) patients. Both IVL and cutting balloon were used in three (5.5%) of these patients. The mean cutting balloon diameter was 3.0 ± 0.2 mm, while the mean IVL balloon diameter was 3.4 ± 0.2 mm.
IVUS was performed in 44 (83.0%) patients. Mechanical support was used in the form of intra-aortic balloon pump in five patients and Impella in one patient. Crossover stenting from LM to LAD or LCX in provisional stent approach was performed in 41 (74.5%) patients, and two-stent LM bifurcation was performed in 14 (25.5 %) patients. The most common technique in the two-stent approach was ‘mini-crush’, which was performed in 12 patients. The mean number of stents per patient was 2.2 ± 0.7. The mean stent length per case was 59.8 ± 20.3 mm. The mean stent diameter of the LM to LAD/LCX stent was 3.51 ± 0.24 mm, while the overall mean stent diameter was 3.03 ± 0.29 mm. The proximal optimisation technique (POT) was performed in 100% of patients, regardless of if they were treated with the one-stent or two-stent approach. The mean POT balloon diameter was 4.5 ± 0.2 mm. The final kissing balloon inflation was performed in all patients treated with the two-stent approach.
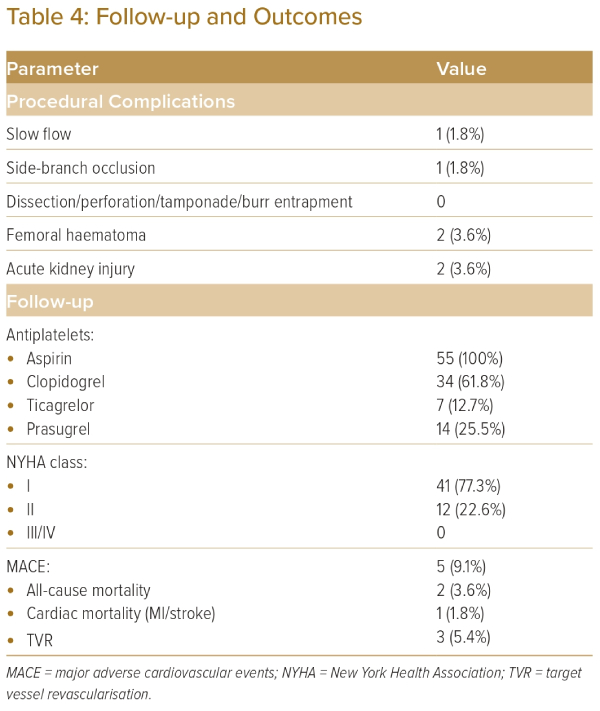
Procedural complications were rare. Slow flow occurred in one patient, which resolved with administration of intracoronary nicorandil and adenosine. A side-branch was occluded transiently in one patient after RA, which was managed by kissing balloon inflation. The angiographic success rate was 100%, with no cases of flow-limiting dissection, perforation, burr entrapment or cardiac tamponade. Angiographic and procedural details are summarised in Table 3. All patients who underwent IVUS guidance met the standard IVUS criteria of stent expansion.11–13 On IVUS, the final post-stenting minimum stent area attained in the ostial LAD was 8.3 ± 0.5 mm2, in the LM was 13.5 ± 0.7 mm2 and in the ostial LCX was 7.1 ± 0.4 mm2.
Outcomes
During hospital stay, local site complications (femoral haematoma) occurred in two (3.8%) patients. No patient developed femoral pseudoaneurysm or retroperitoneal haematoma. Two (3.8%) patients had acute kidney injury, which resolved without the need for haemodialysis. One patient (1.9%) died because of a severe upper gastrointestinal bleed on the second day after the procedure. He was taking aspirin and ticagrelor. No patient required repeat revascularisation during their hospital stay.
Follow-up of the patients was available for a mean of 16.3 ± 11.2 months. Three patients underwent repeat PCI for target lesion revascularisation at the LCX ostium at a mean follow-up duration of 18 months. One patient died 13 months after PCI because of presumed sudden cardiac arrest. He was an elderly man with severe Parkinson’s disease. Further details of this patient were not available. All patients were on regular follow up, and were in New York Health Association class I or II. No patient had subsequent MI. Outcomes are shown in Table 4.
Discussion
Our study demonstrates that RA-assisted PCI for complex and calcified LM disease is safe, with favourable results. The use of concomitant plaque modification therapies under imaging guidance helps in optimisation of the results.
The mean age of our study population was 64.7 ± 9.0 years, younger than similar studies by the Euro4C group, Chiang et al. and Sulimov et al.18,20,22 This could possibly be attributed to the younger age of coronary artery disease in the Indian population, as demonstrated by Iyenagar et al.26 Hypertension and diabetes were the most common comorbidities, with a higher percentage of people with diabetes (43.6%) as compared with the SYNTAX trial (25%), consistent with the disproportionately high prevalence of diabetes in India.6
Our study had almost equal representation of chronic and acute coronary syndrome (ACS), at 45.5 and 54.5%, respectively. This highlights the importance of the need for LM-PCI using RA in the setting of ACS, where the patient may be haemodynamically unstable, unfit for surgery or provision for emergency CABG being unavailable. Other large studies of RA-assisted LM-PCI have also shown 34−82% of such patients presenting with an ACS.18,20 The safety and potential necessity of RA in ACS patients has been previously published.27
Angiographically, LM followed by LAD and LCX were the most common vessels involved. The mean SYNTAX score was 34.9 ± 7.7, higher than the mean SYNTAX score of other similar trials, including the ROTATE registry, and studies by Sulimov et al. and Dahdouh et al., where the mean SYNTAX scores were 30, 28 and 29, respectively.21–23 It was also higher than the SYNTAX score in the EXCEL, SYNTAX and NOBLE trials (mean score 21, 22 and 30, respectively), because of inclusion of only LM patients with significant angiographic calcification.5,6,10
It is to be highlighted that despite CABG being the preferred treatment in patients with such a high SYNTAX score, there are several limitations of CABG in diffuse coronary artery disease and developing countries. In our centre, which is a government tertiary care referral centre catering to a large geographical area and a population of >50 million, the waiting time for CABG for patients can range up to 24 months. Some patients choose not to undergo CABG despite adequate counselling because of personal preferences. A few patients have a diffuse disease and surgeons refuse CABG. Thus, in a resource-limited set-up, such as ours, the SYNTAX score by itself cannot fully account for the decision of CABG versus PCI.
The median burr size was 1.50 mm, which was similar to other recent studies, where it ranged from 1.4 to 1.7 mm.19–23 The mean number of burrs per patient was 1.3, which was lower than the study by Chiang et al. (1.7 burrs per patient), but higher than the studies by Dahdouh et al. (1.1 burrs per patient) and the ROTATE registry (1.2 burrs per patient).20,21,23
A total of 35 patients (63.6%) required the use of additional therapy beyond a single RA burr. This included using an additional (larger-sized) RA burr, cutting balloon and/or IVL. This is expected in large vessels, such as LM and proximal LAD, where a single burr alone may not cause enough plaque modification to ensure adequate stent expansion. The proportion of patients receiving an additional therapy was much higher than that in the RA-assisted LM-PCI subset of the study by Arshad et al., where it was 26.4%.19 Details of using multiple devices in most other studies of calcified LM-PCI are lacking.
The present authors have previously shown the synergistic role of calcium modification, such as RA and IVL, in previous publications.28,29 Upfront RA modifies superficial calcification, and assists in delivery and use of subsequent devices, such as CB/IVL, for plaque modification if required.2,8
IVUS-guided stent expansion criteria were met in 43 out of 46 patients (93.5%) in which IVUS was performed. This is uncommon, as generally they are met in approximately 53–54% of patients in most imaging-guided PCI studies.30,31 This can be due to additional plaque modification devices being used in a majority of patients, and all procedures being performed by the same team with reasonable experience in these procedures.
Procedural complications were low: one patient had side-branch occlusion and one patient had slow flow. No patient had RA-related complications, such as stuck burr or coronary perforation. The incidence of coronary perforation in previous similar studies ranges from 2.3% to 5%.18,19 No perforation in our study may be due to chance and the small number of patients, but could also be due to imaging-based device sizing in 83% of procedures, as compared with 13% in the large Euro4C study and 55% in the ROTATE registry.19,23 Incidentally, one of the patients underwent simultaneous LM-RA and transcatheter aortic valve implantation, which has been previously published by the present authors.32
POT of the LM was performed in 100% of the cases, which led to better outcomes. This is higher than the POT performed in other recent studies of LM-PCI, where it ranged from 81 to 90%.3,4,10,22,33 True LM bifurcation lesions were found in 22 (40%) of our patients. Of these 22 patients, the two-stent approach was performed in only 14 (63.6%), while a provisional one-stent strategy was used in the rest of the patients. This is in concordance with the EBC-Main study, where a single-stent strategy showed a trend of better long-term outcomes.34
MACE in our study was 9.1% at mean follow-up of 16.3 months. This is much lower than MACE in previous studies reporting RA use in LM, where it was between 16 and 38%.18–23 Lower MACE in our study could be due to chance, small sample size, younger study population, IVUS-guided PCI in the majority of patients, use of LM POT in all patients and a final kissing balloon inflation in all cases with two-stent bifurcation stenting.
Limitations
Ours was a single-centre, retrospective study with a relatively small sample size, and the results may not be applicable across different ethnicities and to centres with lesser experience. All PCIs were performed by a single team, which is well experienced in such procedures, which limits the generalisability of our study. Owing to its retrospective nature, it may be associated with bias. Larger, prospective, randomised studies of calcified LM lesions are needed to adequately compare RA-assisted PCI with CABG.
Conclusion
RA-assisted PCI for unprotected calcified LM lesions can be safely performed. An algorithmic approach to calcified lesions is important. Adequate lesion preparation in calcified LM disease frequently requires use of multiple synergistic devices. Imaging-guided PCI increases procedural safety and can lead to satisfactory clinical results in short- to medium-term follow-up. 
Clinical Perspective
- Rotablation-assisted percutaneous coronary intervention of the left main coronary artery performed by an experienced team is safe, with good short- to medium-term outcomes.
- Adequate plaque modification requires the use of adjunct plaque modification therapies, such as a cutting balloon and intravascular lithotripsy with rotablation, in a large proportion of the cases, and proximal optimisation technique is essential for optimum results in all left main percutaneous coronary intervention cases.
- Intravascular ultrasound guidance is an important tool in guiding the management of calcified lesions, especially in the left main artery.
- An algorithmic approach to management of calcified lesions may improve procedural safety and results.