Among patients with cryptogenic stroke, a diagnosis of exclusion, individuals with patent foramen ovale (PFO) constitute a distinct subgroup. The detection of PFO is gaining increasing relevance because of evidence suggesting that PFO-related stroke patients have a reduced risk of recurrent stroke following PFO closure. In 2018, a meta-analysis of four trials (the PC Trial, RESPECT extended follow-up, REDUCE and CLOSE) revealed that PFO closure resulted in a decrease in recurrent stroke risk, falling from 5.1% to 1.8% during follow-up periods from 3.2 to 5.9 years.1–5 This translated to an absolute risk reduction of 3.3% (95% CI [6.2–0.4%]).1–5 In 2021, another meta-analysis of six randomised controlled trials, with a median follow-up period of 4.8 years, established that PFO closure led to a reduced annualised stroke incidence of 0.47%, in contrast with 1.09% with medical therapy alone (adjusted HR 0.41; 95% CI [0.28–0.60]).6
Despite the existing evidence supporting the benefits of PFO closure, the optimal approach to screening for the presence of a PFO remains uncertain. While advanced imaging such as CT and MRI have been proposed as potential tools, their broader availability and sensitivity may be limited compared with ultrasound techniques.7,8 Ultrasound modalities are more cost-effective, readily accessible and are commonly used as the primary diagnostic approach for PFO. Nevertheless, comprehensive data regarding their accuracy have been lacking.
The definitive diagnostic techniques for identifying a PFO involve direct visualisation of the PFO, which can be achieved with transoesophageal echocardiography (TOE) or intra-cardiac echocardiography (ICE).9,10 If available, 3D TOE imaging can be used to improve the visualisation of a PFO.10 However, TOE and ICE are both invasive procedures with significant barriers preventing them from being used routinely as primary screening modalities. TOE has complex infrastructure requirements, and carries the risks of sedation- or anaesthesia-related complications, aspiration and oesophageal trauma.10 In addition, the requirement for sedation or anaesthesia can make efforts to perform the Valsalva manoeuvre suboptimal.11 ICE requires additionally trained personnel, incurs the cost of single-use ICE catheters and carries the risks of vascular complications and arrhythmia.10
Therefore, initial screening for PFO often does not depend on direct visualisation, but instead relies on demonstration of a right-to-left shunt (with or without the Valsalva manoeuvre), in the absence of an otherwise known congenital defect that may permit such a shunt. The existence of a right-to-left shunt can be indicated by the sonographic observation of ‘bubbles’ in the arterial circulation following the injection of agitated saline into a peripheral vein. These bubbles can be visualised in the left atrium or ventricle using transoesophageal echocardiography with bubble (TOE-b) or transthoracic echocardiography with bubble (TTE-b) studies. Alternatively, the bubbles can also be visualised in the middle cerebral arteries (MCA; or other intracranial arteries) using transcranial Doppler with bubble (TCD-b). Given the limitations of TOE-b described above, previous guidelines have suggested a two-of-three approach to screening and confirmation of PFO, with TCD-b and/or TTE-b being employed as the initial screening test(s), and TOE-b being used as the confirmatory test if one of the former two are positive.12 However, the diagnostic accuracy of these tests remains uncertain. Using TOE-b as the reference standard, TTE-b has been reported to have varying sensitivities ranging from 11% to 76%.13–15 Conversely, TCD-b has shown higher sensitivity (94%–100%) than TTE-b, although its limitation in anatomical localisation of the shunt may be disadvantageous.16,17
Although the diagnostic accuracy of TCD-b and TTE-b has been studied, most investigations evaluate each modality in isolation, introducing the possibility of inherent study bias affecting the outcomes. Limited studies have evaluated both modalities simultaneously. In a study involving 32 patients where TCD-b, TTE-b and TOE-b were performed simultaneously, TCD-b exhibited a sensitivity, specificity, and accuracy of 100% for predicting an interatrial right-to-left shunt as determined by TOE-b. In contrast, TTE-b demonstrated a sensitivity of 54%, specificity of 94% and accuracy of 77%.18 In a separate study, TCD-b and TTE-b demonstrated higher sensitivities (97% and 100%, respectively) compared with TOE-b (86%) in demonstrating right-to-left shunt.19
Simultaneously performing TCD-b and TTE-b enables the exploration of concordance (both TCD-b and TTE-b yielding positive or negative results) and discordance (TCD-b yielding positive results while TTE-b yields negative results or vice versa) rates for each individual patient. Analysing the prevalence and factors related to concordance and discordance between TCD-b and TTE-b outcomes has the potential to guide individualised approaches in selecting the optimal screening test for PFO, maximising the diagnostic yield in each patient.
Therefore, the main aim of our study was to assess the rates of concordance and discordance between TCD-b and TTE-b when conducted simultaneously.
Methods
This was a prospective observational study of patients who presented with ischaemic stroke or transient ischaemic attack (TIA) between 1 June 2018 and 30 June 2021. It was a single-centre study in a major national referral hospital benefitting from the comprehensive provision of acute stroke care services, encompassing IV thrombolysis and endovascular therapy.
The study included patients who experienced a stroke or TIA, had a risk of paradoxical embolisation (RoPE) score of ≥6 and for whom TCD-b and TTE-b examinations were requested by the treating medical team.
Patients were excluded if they had a history of permanent or paroxysmal AF, presence of left-sided intra-cardiac thrombus, prosthetic heart valves, atrial myxoma or other cardiac tumours, moderate or severe mitral stenosis, recent MI (within the preceding 4 weeks), left ventricular ejection fraction <35%, moderate or severe valvular regurgitation or active endocarditis. Patients with extra-cranial or intra-cranial atherosclerosis with ≥50% luminal stenosis in arteries supplying the ischaemic region were also excluded.
Enrolled patients underwent simultaneous TCD-b and TTE-b in the neuro or cardiovascular laboratory. All patients had a TTE performed first. If this was normal, patients then proceeded to concurrent TCD-b and TTE-b studies. The studies were performed as per recommended consensus, with some considerations as described here.20 For TCD-b studies, both (bilateral) MCAs were insonated. If the MCA could not be insonated, the ipsilateral posterior cerebral artery (PCA) was insonated instead. Ideally, an 18 G IV cannula over the right antecubital fossa was preferred for injection. However, if this venous access was challenging, an 18 G or 20 G IV cannula sited over, in order of preference, the right forearm, right wrist, left antecubital fossa, left forearm or left wrist was used instead. Siting an IV cannula anywhere other than the upper limbs was not permitted. To optimise TTE images, all studies were performed with patients’ chest laterally rotated to the left side at an angle of approximately 30° to the horizontal. Patients’ heads remained supine. Agitated saline was prepared by manual mixing of 8 ml of 0.9% sodium chloride with 1 ml air and 1 ml of blood drawn from the IV cannula. If blood could not be aspirated from the cannula, 9 ml of 0.9% sodium chloride with 1 ml of air was mixed instead. Saline agitation and injection of agitated saline was conducted at the patient’s bedside under the supervision of a physician. IV agitated saline was administered once while the patient was at rest and at least twice during a Valsalva manoeuvre. Prior to the injection of agitated saline, patients were instructed on how to properly perform the Valsalva manoeuvre. The Valsalva manoeuvre began when the contrast filled the right atrium and was sustained for a duration of 5 seconds. The effort of Valsalva manoeuvre was categorised as good if it resulted in approximately one-third reduction in mean intracranial arterial flow velocities on TCD or if there was evidence of the interatrial septum moving towards the left atrium on TTE. A Valsalva attempt was considered poor if there was no change in intracranial flows or interatrial septal movement. An intermediate effort lying between these two descriptions was graded as fair. If at least one of the efforts was judged to be poor, an additional attempt was repeated.20
TCD studies were performed with the SONARA TCD machine (Natus Medical), with the settings optimised for high intensity transient signals (HITS) detection. Either CX50 (Philips) or S70 (GE HealthCare) echocardiography machines were used for TTE-b examinations, with the use of harmonic imaging when available. The acquisition of apical four-chamber or modified four-chamber echocardiography views allowing for proper visualisation of all four cardiac chambers and the interatrial septum was obtained for assessment.
For the TCD-b studies, bubbles were defined as HITS fulfilling the definition of microembolic signals (MES).21 The total number of bubbles in both MCAs or PCAs counted from the attempt that produced the maximal number within 40 seconds after injection was used to grade the severity of the shunt as small (1–10 bubbles corresponding to international consensus grade 2), moderate (>10 bubbles corresponding to international consensus grade 3) or large (>10 bubbles with curtain effect corresponding to international consensus grade 4).20 The latency time (LT) between the injection and the appearance of the first bubble was also recorded.
For the TTE-b studies, the presence of bubbles was assessed visually. The quantification of shunt severity was based on the maximum number of bubbles counted in the left atrium in a single frame, and categorised as small (1–9 bubbles), moderate (10–20 bubbles) or large (>20 bubbles).22,23 The observation of left atrial bubbles occurring ‘early’ (<5 cardiac cycles) subsequent to the release of the Valsalva manoeuvre was interpreted as evidence of intra-cardiac shunting, while the appearance of bubbles ‘late’ (≥5 cardiac cycles) was taken as an indication of extra-cardiac shunting.22
For this study of simultaneously performed TCD-b and TTE-b, TOE-b was not part of the study protocol. However, if TOE-b was subsequently performed as part of clinical care, data from TOE-b were also collected, and shunt severity was quantified using the same criteria for TTE-b above.23
Data were analysed using SPSS Version 26.0 (IBM). Non-parametric tests were used for analysis. Continuous variables were expressed as median (25th percentile, 75th percentile). Comparison of medians between two groups was analysed using the Mann–Whitney U-test for non-normally distributed data, and Fisher’s exact test for categorical variables with groups of frequency <5.
Results
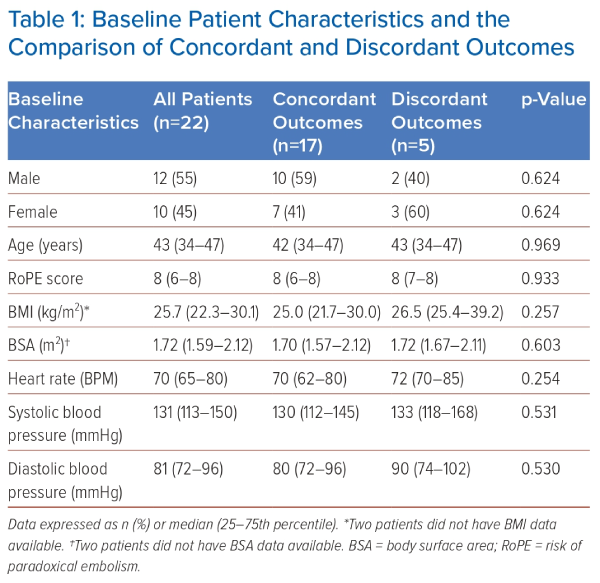
The study comprised 22 patients, of whom 12 (55%) were male. Of note, recruitment was affected by the COVID-19 pandemic. The median age of the cohort was 43 years (Table 1). No patient had an atrial septal defect. Median RoPE score was 8 (Table 1). All patients successfully attained at least a fair level of effort during the Valsalva effort, and a notable 21 out of 22 patients accomplished a good Valsalva effort.
Twenty-one individuals had satisfactory bilateral temporal windows for bilateral MCA insonation. One patient had an inadequate right temporal window, and her right PCA was insonated with her left MCA.
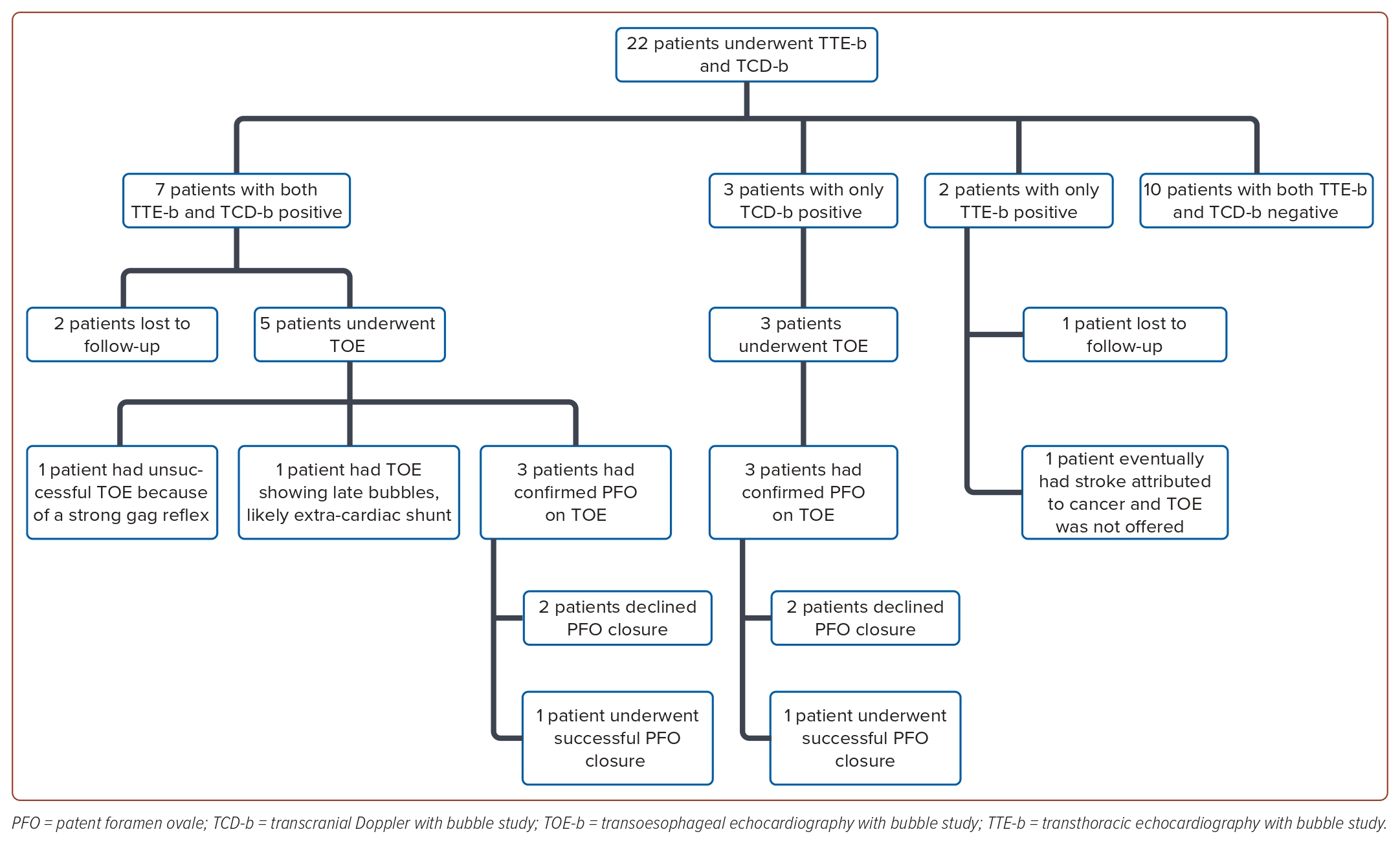
Seventeen patients (77%) demonstrated concordant outcomes between their TCD-b and TTE-b studies. Among these patients, 10 (45%) displayed concordantly negative results, while seven (32%) exhibited concordantly positive outcomes. Discordant findings were observed in five patients (23%), with three showing positive TCD-b results but negative TTE-b results, and two showing negative TCD-b results with positive TTE-b results. Figure 1 details the breakdown of the patients by TCD-b and TTE-b results.
Concordant Group
Seven patients exhibited concordantly positive results in both TCD-b and TTE-b studies. Of these seven patients, only three had shunts (all small) at rest on TCD-b and two patients had shunts (both small) at rest on TTE-b, but all seven patients turned concordantly positive upon Valsalva manoeuvre (Table 2). On TCD-b, the LT ranged from 1 to 9 seconds. Five of all seven patients underwent TOE-b, while two were lost to follow-up.
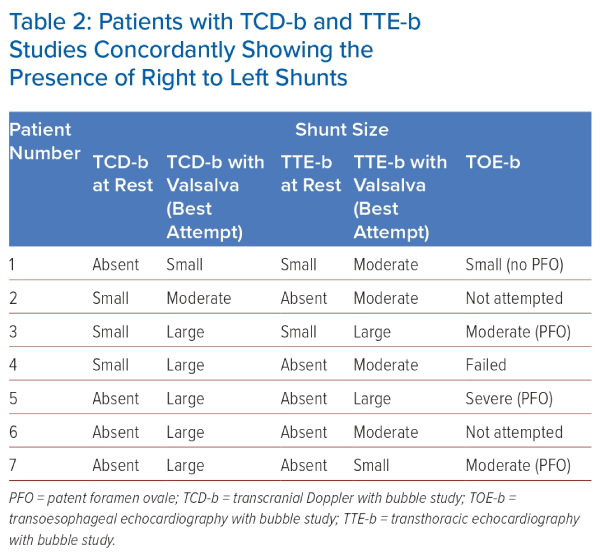
Among the five patients who underwent TOE-b, four were confirmed to have a right-to-left shunt, whereas one patient’s procedure was unsuccessful because of a strong gag reflex. Among the four patients with confirmed shunt, three were identified to have a PFO through direct visualisation on TOE-b. While all three of these patients had large shunts on TCD-b, TOE-b showed moderate shunts in two and a large shunt in one. In the fourth patient with confirmed shunt, no PFO was observed, and the appearance of bubbles occurred ‘late’ (after 5 cardiac cycles), implying the presence of an extra-cardiac shunt. This patient’s TCD-b study had the smallest shunt recorded in our study, with only two bubbles seen on the second Valsalva manoeuvre, as well as the joint-longest LT of 9 seconds. Her TTE-b study recorded a moderate but late shunt.
Among the three patients with confirmed PFO, the TCD-b study yielded a higher number of bubbles compared with TTE-b. Specifically, TCD-b indicated the presence of >30 bubbles with curtain effect in all three cases (indicating a large shunt), whereas TTE-b showed 20 bubbles in two cases (large shunt) and five bubbles in one case (small shunt).
Ten patients demonstrated concordantly negative results in both TCD-b and TTE-b studies. All these patients exhibited good Valsalva efforts. However, none of them underwent subsequent TOE-b evaluation.
Among all patients with concordantly positive or concordantly negative results, the median BMI was 25.0 kg/m2 (21.7–30.0). The median values for other variables such as the RoPE score, BSA, heart rate, systolic blood pressure and diastolic blood pressure are shown in Table 1.
Discordantly Positive Transcranial Doppler-bubble Group
Three patients had positive TCD-b results but negative TTE-b studies. Within this subset, two patients displayed large right-to-left shunts on TCD-b upon Valsalva manoeuvre, with more than 50 bubbles along with curtain effect (one of whom had a mild shunt already detectable at rest; the other had no shunt at rest). Another patient exhibited moderate right-to-left shunt with up to 14 bubbles upon Valsalva manoeuvre, but no shunt at rest. The LT for these three patients ranged from 6 to 9 seconds. All three underwent TOE studies, which confirmed the presence of PFO with right-to-left shunt. One of these three patients had a large shunt on both TCD-b and TOE-b, another had a moderate shunt on TCD-b but large shunt on TOE-b, and the last patient had a large shunt on TCD-b and moderate shunt on TOE-b.
Discordantly Positive Transthoracic Echocardiography-bubble Group
Additionally, there were two patients with negative TCD-b results but positive TTE-b results, neither of whom underwent subsequent TOE. Specifically, one of them had a mild right-to-left shunt with five bubbles on TTE-b detected only on Valsalva manoeuvre but not detected at rest (this patient was lost to follow-up), while the other patient exhibited a large right-to-left shunt (>20 bubbles) both at rest and with Valsalva manoeuvre. Interestingly, in the latter patient, bubbles appeared ‘late’ at rest (after nine cardiac cycles). On Valsalva manoeuvre, her bubbles appeared ‘early’ but then persisted beyond five cardiac cycles. This patient was subsequently diagnosed with metastatic ovarian adenocarcinoma and her stroke aetiology was eventually attributed to cancer-related causes rather than a PFO, hence TOE-b was not offered. On PET scan, the sites of metastases included pleura and a cardiophrenic lymph node, but there was no obvious cardiac focus of metastasis. Given that cancer-associated right-to-left shunts (both intra-pulmonary and intra-cardiac) have been described before, we wonder if her unusual TCD-b and TTE-b findings may be related to undetected cancer-associated shunt(s), with or without an associated underlying PFO.24,25
The patients with discordant outcomes had a trend towards a higher median BMI (26.5 kg/m2 [25.4–39.2]), compared with the median BMI of patients with concordant outcomes, although the difference did not reach statistical significance (U=20.0; p=0.257; Table 1). Median values for other variables, with the respective U statistic and p-values when analysed against concordance and discordance, are shown in Table 1.
Discussion
The findings of our study underscore a significant rate of discordance (23% of cases) between TCD-b and TTE-b studies. Our investigation revealed that, in terms of identifying right-to-left shunts, TCD-b may exhibit better predictive capability for TOE-b outcomes compared with TTE-b. Across both concordant and discordant groups, seven patients underwent successful TOE-b examinations, and TCD-b accurately anticipated a right-to-left shunt on TOE-b in all seven patients, six of whom had PFOs while one presented an extra-cardiac shunt rather than a PFO. The patient with an extra-cardiac shunt was also potentially identifiable on TCD-b by means of a low number of bubbles and long LT, as this combination has previously been reported to have a lower association with PFO.26 In contrast, TTE-b was negative in four of these seven patients. Although the number of patients in our study was small, the data may provide some support for earlier research suggesting that TCD-b may be the superior screening method for detecting PFO.18
TCD-b, TTE-b and TOE-b use ultrasound, hence the quality of these studies would be affected by patient factors, such as poor temporal windows and dermal thickening because of fibrosis, scarring or other factors. Although statistical significance was not achieved (because of the small numbers), the observed variation suggests that a high BMI may adversely impact the sensitivity of TTE-b more than TCD-b. This may be because BMI values are associated with greater variations in chest wall thickness than in scalp thickness and higher chest wall thicknesses adversely affect the quality of cardiac insonation.27–29 This phenomenon stems from the limitations posed by increased adipose tissue layers, which can attenuate ultrasound waves and hinder the quality of echocardiographic imaging. Furthermore, cardiac MES are identified visually, without an audible component that can be heard for MES on TCD-b, which can compromise the sensitivity of TTE-b. Conversely, TCD-b relies on ultrasound beam penetration of the skull, using four main acoustic windows in clinical practice: transtemporal, transorbital, suboccipital and submandibular windows.30 Typically, the transtemporal bone window serves as the established choice for TCD examination in adults. Although scalp thickness varies with BMI, TCD performance may be more contingent on the thickness of the temporal bone in the plane of the artery being studied rather than scalp thickness.31 Our study included 22 patients undergoing TCD-b, among whom only a single patient encountered challenges with insonation of the right MCA using the temporal window. Consequently, insonation of the right PCA through the same temporal window was necessary. This suggests a considerable success rate achieved in TCD-b examinations across a population with varying BMI values.
Throughout the study, we consistently observed that the Valsalva manoeuvre improved the sensitivities of both TCD-b and TTE-b, contrasting with examinations conducted at rest. There were more bubbles on Valsalva manoeuvre than at rest for all patients. The Valsalva manoeuvre’s propensity to improve sensitivity in detecting right-to-left shunts is well-established; thus, the quality of Valsalva effort should be carefully evaluated, and additional attempts should be made if initial efforts appear suboptimal.32,33
TCD-b has demonstrated comparable sensitivity and specificity to TOE in detecting right-to-left shunting.34 Notably, TCD-b presents several advantages, including its cost-effectiveness, repeatability and non-invasiveness. Our findings provide some support for the American Association of Neurology’s advocacy of TCD-b as the preferred screening method for evaluating right-to-left shunt in patients with suspected PFO-associated CVA.35 Therefore, our suggestion is that, when presented with a suspected PFO-associated stroke scenario, TCD-b should be prioritised as the primary screening tool for detecting right-to-left shunting. Nonetheless, it is important to emphasise that TCD-b does not replace TTE in the evaluation for embolic sources of stroke other than PFO, nor the necessity of TOE in cases where a further search for embolic sources is required.
By performing both TCD-b and TTE-b simultaneously in our patients, we were able to exclude certain potential confounders (such as variation in the IV cannula site or agitated saline protocol) as potential sources of the discordance. Consequently, we can conclude that, within our patient cohort, discordance primarily stemmed from inherent limitations of each test. However, the main limitation of our study is the small sample size, preventing us from drawing any definitive and precise conclusions about the sources of discordance. In addition, only seven of our 22 patients underwent TOE-b examinations, restricting our ability to further explore the concordance and discordance of TCD-b and TTE-b in relation to TOE-b. Finally, another potential limitation comes from the fact that upper-extremity veins were used for injection of agitated saline, because there have been suggestions that injection via lower-extremity veins may provide better assessment of the interatrial septum.36
Conclusion
In this study, we describe our experience in simultaneous TTE-b and TCD-b examinations in patients with suspected PFO-associated stroke. A notable discordance rate was observed between TCD-b and TTE-b outcomes. Our study also raises the possibility that, compared with TTE-b, the sensitivity of TCD-b may be less affected by a high BMI value and that TCD-b may be a better screening test for PFO. 
Clinical Perspective
- This study identified that, in screening for the presence of a patent foramen ovale, there was a significant discordance rate (23%) between the results of transcranial Doppler with bubble (TCD-b) and transthoracic echocardiography with bubble (TTE-b) studies.
- Such discordance is primarily because of inherent limitations of each screening modality.
- A high BMI value may adversely affect the sensitivity of TTE-b more than TCD-b.
- Although TCD-b cannot replace transthoracic echocardiography in the evaluation of embolic sources of stroke, TCD-b may be an overall better screening test than TTE-b for the detection of patent foramen ovale.