COVID-19, caused by severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2), has resulted in more than 6 million deaths worldwide in 226 countries as of 13 April 2022.1 Patients with cardiometabolic risk factors (CMRF) have an increased risk of developing severe COVID-19 disease and mortality. Emerging literature has shown that patients with CMRF have an increased angiotensin-converting enzyme 2 (ACE2)-dominant expression, promoting SARS-CoV-2 entry, which already has a tropism for the heart and lungs. This is further compounded by the use of ACE inhibitors and angiotensin-receptor blockers in such patients.2 As a result, COVID-19 disease and severe myocardial damage from multiple signalling pathways have been implicated.3,4
Asia is the largest continent in the world, consisting of a heterogenous amalgamation of high and low-middle income countries (LMIC), with widely diverse impacts of COVID-19 disease owing to differences in infrastructure, testing, isolation efforts and economic sufficiency.5 Previous meta-analyses have explored the impact of CMRF on mortality and severe COVID-19 disease; however, they predominantly comprise studies from China, the US and Europe.6–8 These countries are deemed as upper-to-high income economies, and reported significantly higher COVID-19 prevalence and death compared to lower income economies.9 Reasons for this include greater detection, higher transparency of data, as well as greater prevalence of CMRF in the higher income countries. To the best of our knowledge, no study has investigated the differential impact of COVID-19 among patients with CMRF between high-income countries and low-middle income countries in the Asia-Pacific region. The primary objective of the review is to consolidate available evidence in the Asia-Pacific region for patients with both COVID-19 infection and CMRF and its association with mortality and intensive care unit (ICU) admission.
Methods
Our PICOS criteria for the systematic review were as follows: P (Population): adult population in the Asia-Pacific region with confirmed COVID-19 based on reverse-transcription (RT)-PCR testing in both inpatient and outpatient setting; I (Intervention/exposure): CMRF comprising diabetes, hypertension, cardiovascular disease, obesity, smoking and hyperlipidaemia; C (Comparator): patients without the above conditions; O (Outcome): all-cause mortality, ICU admission rates; and S (Study type): cohort studies only.
Search Strategy
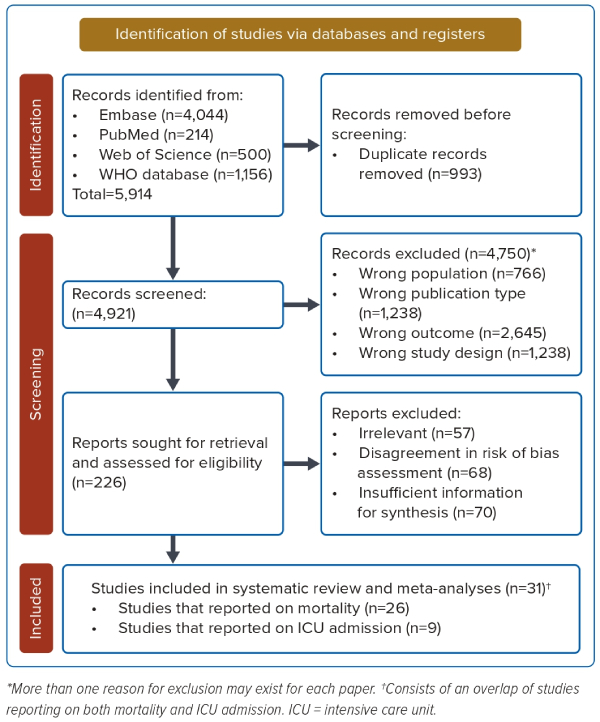
This meta-analysis was performed according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses 2020 statement.10 The review protocol was registered and published on PROSPERO (ID: CRD42022292073) on 4 January 2022. Studies were selected according to a three-step process carried out by two independent reviewers (RYF and AL). A comprehensive search was performed using the following electronic bibliographic databases: MEDLINE, Embase, Web of Science, and the WHO COVID-19 global literature on coronavirus disease. The search strategy is depicted in Figure 1. The database search strategy and query is found in Supplementary Material Table 1.
Study Selection
One author (RYF) conducted the literature search on 30 May 2021 and included all relevant publications without date restrictions. Duplicates were removed and eligible publications were exported to Rayyan, a platform for literature screening. Two reviewers (RYF and AL) independently screened titles and abstracts. All disagreements were resolved by consensus. The inclusion criteria consisted of prospective, cross-sectional and retrospective cohort studies in adult patients with COVID-19 in the Asia-Pacific region. The primary outcome of interest was all-cause mortality and disease severity, defined by ICU admission. Abstracts, letters, review articles, consensus, editorials, correspondence, case series, case reports, opinions, updates, secondary literature and bench-side research were excluded.
Data Extraction and Quality Assessment
Two reviewers (RYF and AL) independently extracted data using a piloted spreadsheet that consisted of the first author, year of publication, study country and region, study design, period of enrolment, total sample size, demographic data (age, number of males) number with the following cardiovascular risk factors: diabetes, hypertension hyperlipidaemia, obesity, smoking and cardiovascular disease. In this study, we defined cardiovascular disease as limited to coronary artery disease and conducted a subgroup analysis to exclude papers that encompassed cerebrovascular diseases and heart failure within their definition. Data on total mortality and severity of disease expressed by ICU admission rates were also retrieved. Disagreements were resolved between the two reviewers. The methodological quality of included studies was reviewed independently by the two reviewers (RYF and AL) using the Newcastle-Ottawa Quality Assessment Form for Cohort Studies.11 The overall risk of bias was based on three domains of bias. Studies were defined as high quality if a consensual rating of at least 7 out of 9 was attained. Discrepancies were resolved through consensus between the two reviewers.
Statistical Analysis
Effect estimates were presented as odds ratio (OR) with a 95% CI. Cochran’s Q χ2 test and the Higgins I2 statistic were employed to quantify the level of heterogeneity across studies. I2 values <25% indicated low, 25–70% indicated moderate and ≥70% suggested high heterogeneity.12 Given the heterogeneity between studies, a random-effects model was employed to estimate the average effect and its precision. Visual inspection of funnel plots and formal testing with Egger’s regression test was used to evaluate the risk of publication bias.13 Univariate and multivariate meta-regression analysis was also performed for age, sex and regional differences to assess whether the CMRF associations were independent and to address heterogeneity among the included studies. All analyses were carried out using R. p<0.05 was considered statistically significant.
Results
Study Selection
A total of 5,914 studies were identified initially, with 4,921 studies extracted following duplicate removal. Of these, 4,750 studies were excluded at the title and abstract screening phase due to them being of either wrong population, publication type, outcome, or study design. Of the remaining 226 studies reviewed for full-text screening, 57 studies were excluded for being out of topic, 68 studies did not achieve sufficient risk of bias assessment criteria (had a score of less than 6), and 70 studies were excluded for having insufficient information for synthesis, yielding 31 studies with 84,011 patients for final analyses (Figure 1).
Study Characteristics
The baseline demographics of the study populations, where available, are reported in Supplementary Material Table 2. Among the 31 studies, 14 (45%) studies were conducted in northern Asia (seven from China, six from South Korea, one from Japan), 16 (51.6%) studies were conducted in South Asia (eight from Iran, four from India, two from Bangladesh, one from Abu Dhabi, United Arab Emirates, one from Pakistan), and one (3.2%) study was conducted in Southeast Asia (Indonesia). Indonesia was subsequently included as part of the analysis with the rest of the South Asian countries. There were 39,741 patients from northern Asia and 44,270 from South Asia. The mean age ranged from 45.3 to 61.8 years, while the median age ranged from 37 to 60 years; 35.8–90.4% of patients were men. Most study designs were retrospective (n=27; 87.1%), followed by prospective (n=3), and one (3.2%) was cross-sectional. Twenty-six studies evaluated the risk of patients’ mortality based on the presence of CVMF, while 10 evaluated the severity of COVID-19 disease resulting in ICU admission (Supplementary Material Table 2).
Pooled Mortality and Intensive Care Unit Admission Incidence Rates
The overall pooled mortality rate was 9.4% (95% CI [6.5–13.3%]) (Supplementary Material Table 3, Supplementary Material Figure 1). The overall ICU admission rate was 9.6% (95% CI [6.3–14.3%]) (Supplementary Material Table 3, Supplementary Material Figure 2). The overall mean or median age was not reported in two studies14,15 and were excluded from the age meta-regression analyses.
Pooled Prevalence of Cardiometabolic Risk Factors
The pooled prevalence rate for diabetes was 18.2% (95% CI [14.0–23.2%]), hypertension 20.6% (95% CI [15.5–27.0%]), cardiovascular disease 9.5% (95% CI [6.3–13.9%]), obesity 9.5% (95% CI [6.0–14.7%]), history of smoking 7.0% (95% CI [3.0–15.4%]) and hyperlipidaemia 2.1% (95% CI [0.4–10.2%]) (Supplementary Material Table 3, Supplementary Material Figures 3–8).
Diabetes
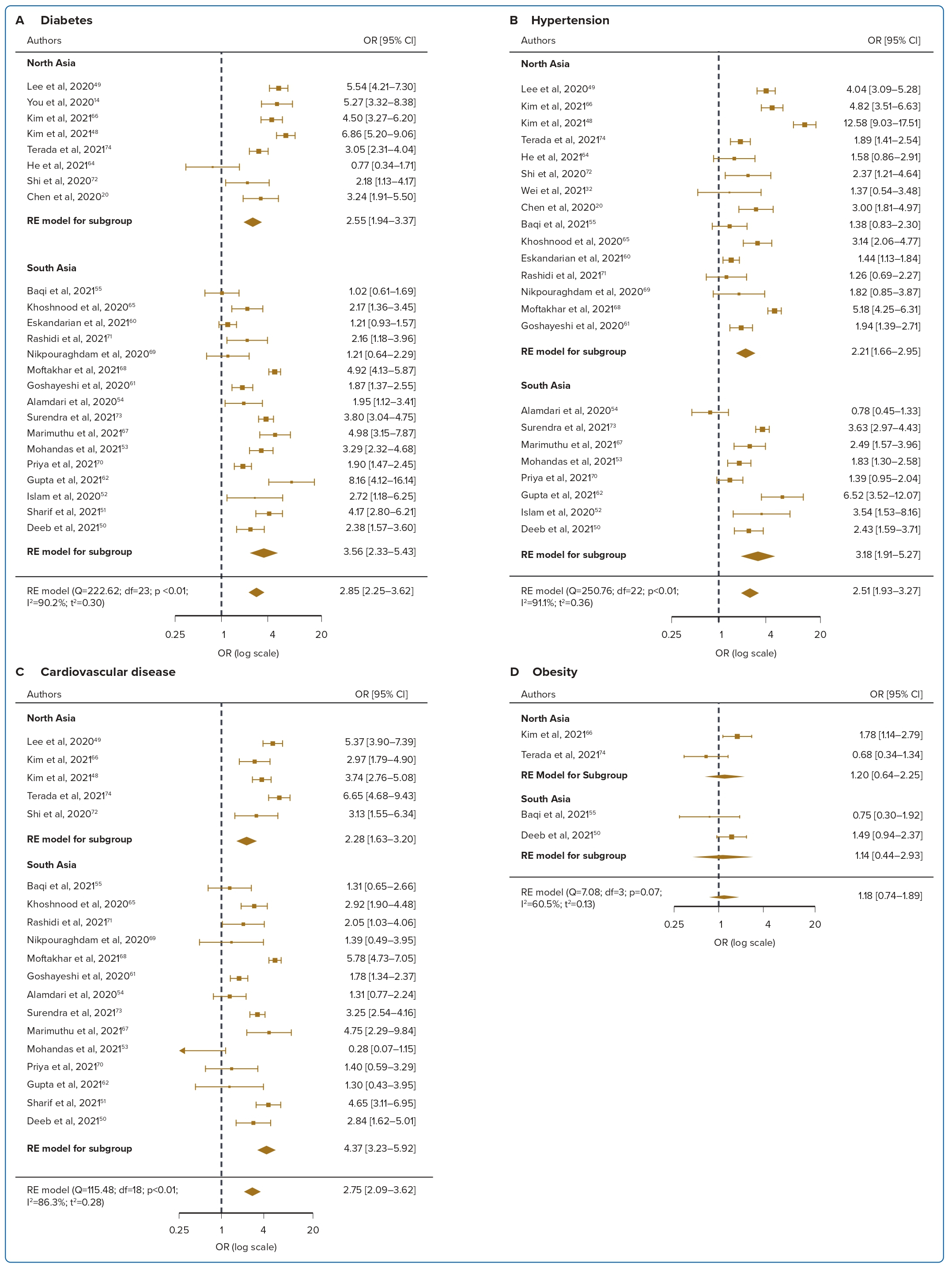
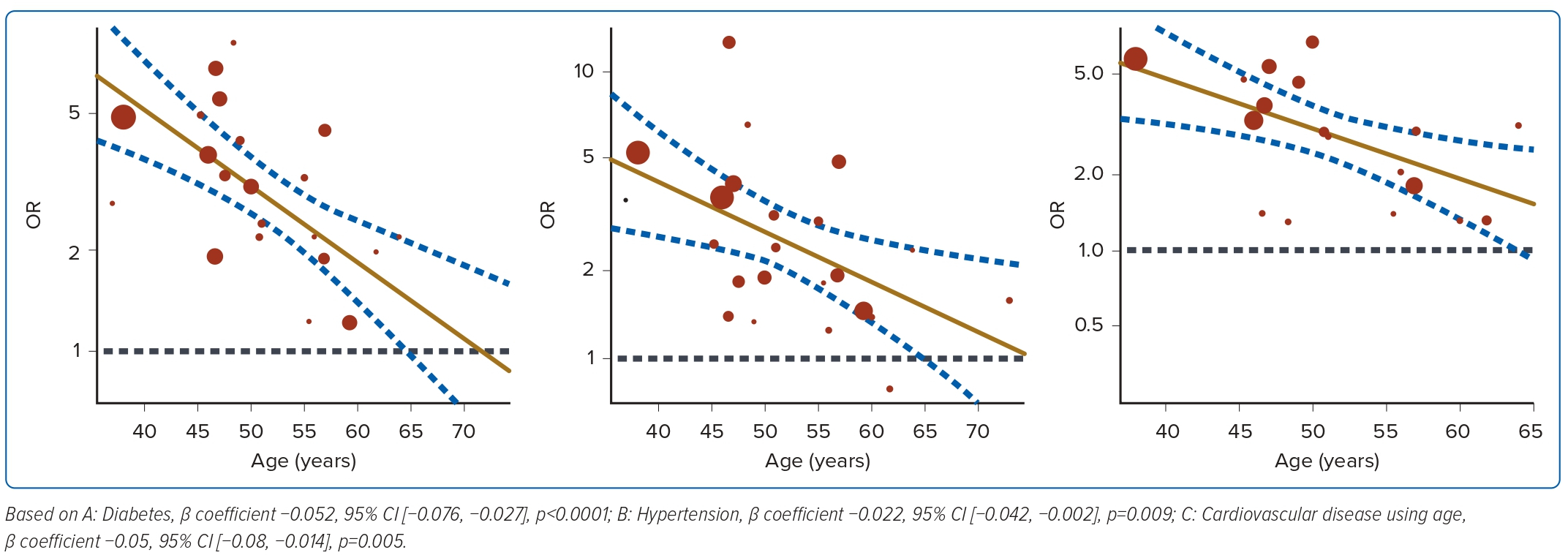
Diabetes was reported in 24 studies for mortality and was associated with a 2.85-fold significant increase in the risk of mortality compared with patients without diabetes, with high heterogeneity (OR 2.85; 95% CI [2.25–3.62]; p<0.001; I2=90.2%; Figure 2A). Nine studies reported diabetes was associated with a 2.86-fold significant increase in the risk of ICU admission (OR 2.86; 95% CI [1.76–4.64]; p<0.001; I2=90.2%; Figure 3A). Univariate meta-regression and sensitivity analyses (Figure 4, Supplementary Material Table 6) demonstrated that the association between diabetes and mortality was influenced by sex (coefficient: −0.020; 95% CI [−0.038, −0.003]; p<0.025) and age (coefficient: −0.052; 95% CI [−0.076, −0.027]; p<0.0001) but was not influenced by regional differences (coefficient: 0.34; 95% CI [−0.15, 0.83]; p=0.18). Further multivariate regression showed that sex (coefficient: −0.017; 95% CI [−0.030, −0.004]; p=0.013) and age (coefficient: −0.049; 95% CI [−0.071, −0.028]; p<0.0001) were both significant predictors of mortality in patients with diabetes (Figure 4A).
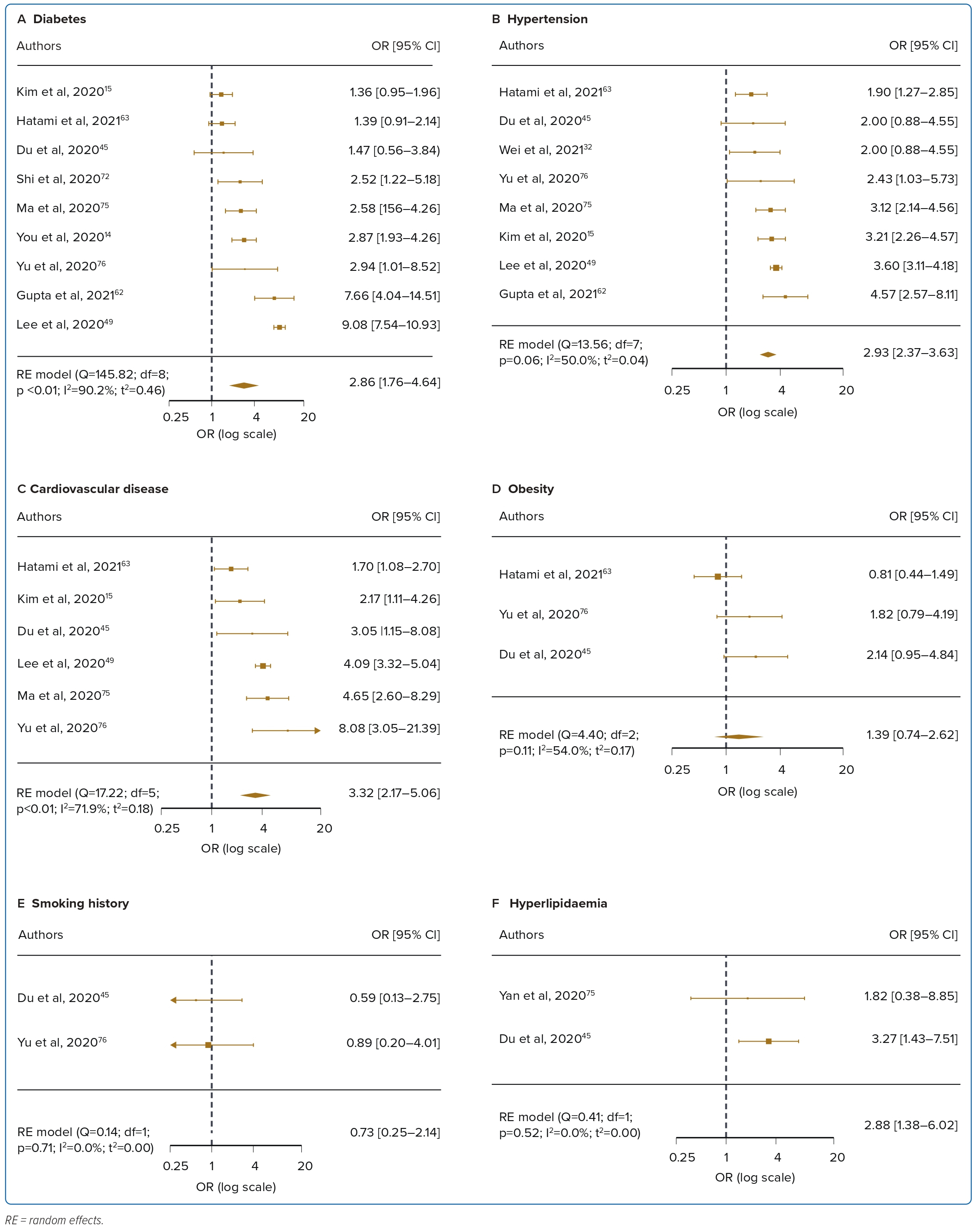
Hypertension
Hypertension was reported in 23 studies for mortality and was associated with a 2.5-fold significant increase in the risk of mortality, with high heterogeneity (OR 2.51; 95% CI [1.93–3.27]; p<0.001; I2=91.1%; Figure 2B). Eight studies reported ICU admission and hypertension was associated with a 2.93-fold significant increase in the risk of ICU admission (OR 2.93; 95% CI [2.37–3.63]; p<0.001; I2=50.0%; Figure 3B). Univariate meta-regression analyses for hypertension indicated that the association between hypertension and mortality was similarly influenced by sex (coefficient: −0.022 95% CI [−0.042, −0.002]; p=0.031) and age (coefficient: −0.04; 95% CI [−0.069, −0.010]; 0.009) but not regional differences (coefficient: 0.37; 95% CI [−0.17, 0.92]; p=0.18). Further multivariate regression showed that sex (coefficient: −0.020; 95% CI [−0.037, −0.0026]; p=0.024), and age (coefficient: −0.037; 95% CI [−0.064, −0.0099]; p=0.0007) were both significant predictors of mortality in hypertensive patients (Figure 4B, Supplementary Material Table 6).
Cardiovascular Disease
Cardiovascular disease was reported in 19 studies and was associated with a 2.75-fold significant increase in risk of mortality, with high heterogeneity (OR 2.75; 95% CI [2.09–3.62]; p<0.001; I2=86.3%; Figure 2C). Six studies reported ICU admission and cardiovascular disease was associated with a 3.32-fold significant increase in the risk of ICU admission (OR 3.32; 95% CI [2.17–5.06]; p=0.004; I2=71.9%; Figure 3C). Univariate meta-regression analyses for cardiovascular disease indicated that the association between cardiovascular disease and mortality was influenced by age (coefficient: −0.05 95% CI [−0.08, −0.014]; p=0.005) and regional differences (coefficient: 0.60; 95% CI [0.05–1.16]; p=0.033) but not sex (coefficient: −0.02; 95% CI [−0.04, 0.0002]; p=0.052). Further multivariate regression showed that age (coefficient: −0.05; 95% CI [−0.08, −0.03]; p<0.0001) and location (north versus south Asia) (coefficient: 0.57; 95% CI [0.24–0.90]; p=0.0007) were both significant predictors of mortality in patients with cardiovascular disease (Figure 4C,Supplementary Material Table 6). Several papers included cerebrovascular diseases and heart failure as part of their definition of cardiovascular diseases. A subgroup analysis was performed to exclude these papers and found no difference in the results, with the OR slightly decreased to 2.33 (OR 2.33; 95% CI [1.73–3.16]; p<0.001; I2=83.73%).
Obesity, Smoking and Hyperlipidaemia
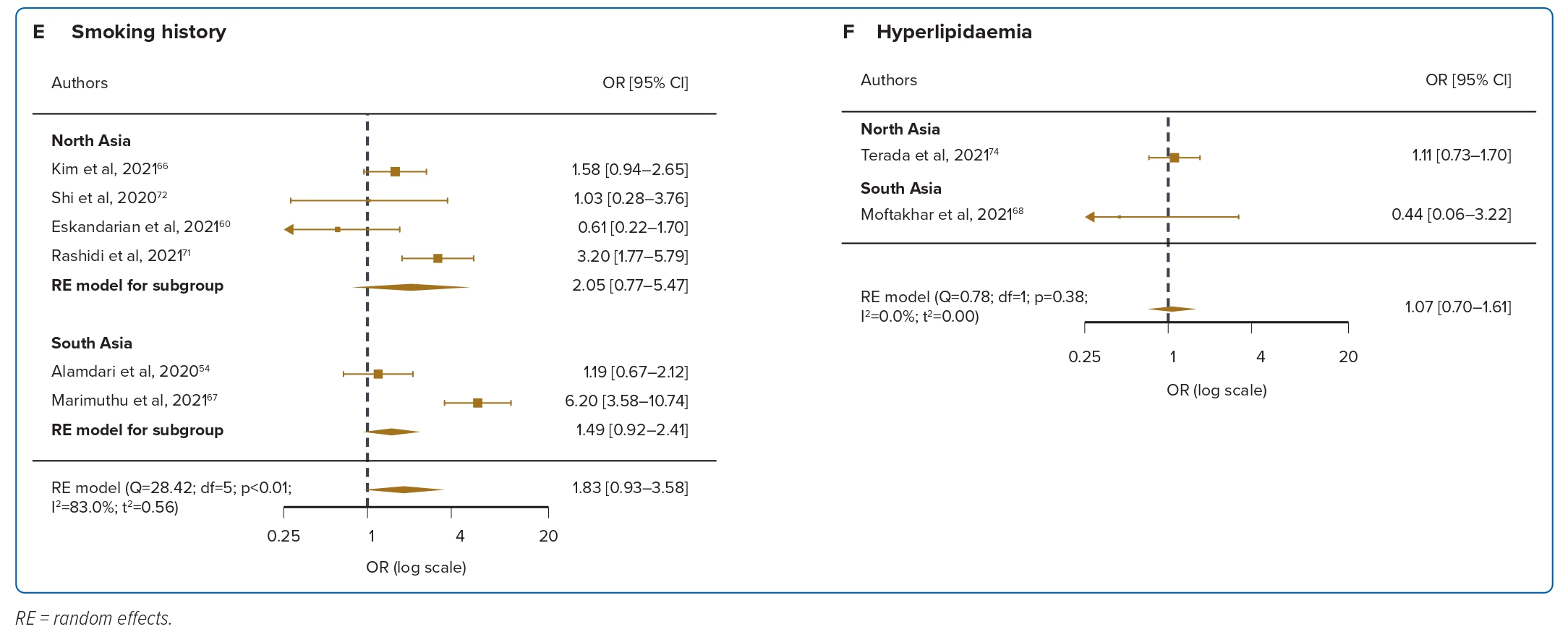
Obesity was reported in four studies for mortality, and was not significantly correlated with mortality, with moderate heterogeneity (OR 1.18; 95% CI [0.74–1.89]; p=0.07; I2=60.5%; Figure 2D). For the three included studies in ICU admission, obesity was significantly associated with ICU admission (OR 1.39; 95% CI [0.74–2.62]; p=0.11; I2=54.0%; Figure 3D). Smoking was reported in six studies and was not significantly correlated with mortality, with low levels of heterogeneity (OR 1.83; 95% CI [0.93–3.58]; p<0.001; I2=83.0%; Figure 2E). For the two included studies in ICU admission, smoking was not significantly associated with ICU admission (OR 0.73; 95% CI [0.25–2.14]; p=0.71; I2=0%; Figure 3E). Hyperlipidaemia was reported in two studies for mortality and was not significantly correlated with mortality, with low levels of heterogeneity (OR 1.07; 95% CI [0.70–1.61]; p=0.38; I2=0%; Figure 2F). For the two included studies in ICU admission, hyperlipidaemia was not significantly correlated with ICU admission (OR 2.88; 95% CI [1.38–6.02]; p=0.52; I2=0%; Figure 3F). Meta-regression analysis was not performed due to the small number of studies in these risk factors.
Quality Assessment
A full risk of bias assessment is presented in Supplementary Material Table 4. The Newcastle-Ottawa Scale ranges from 4 to 9, with the highest score at 9. Every article included had a minimum risk of bias score of 7, indicating low risk of bias and good quality according to Agency for Healthcare Research and Quality standards.11
Heterogeneity
Meta-regression showed that the patients’ age, sex, and regional differences were important sources of heterogeneity when examining the potential effect of CMRF on mortality.
Publication Bias
We assessed publication bias for the risk factors for mortality (diabetes, hypertension and cardiovascular disease) that had at least five studies. The funnel plots indicated no publication bias was present for diabetes and hypertension; however, publication bias was likely for cardiovascular disease in view of larger studies with a positive effect.
Visual inspection of the standard error Egger plots for the mortality analysis (Supplementary Material Figure 9) suggested symmetry without an underrepresentation of studies of any precision for studies reporting on diabetes and hypertension. Egger’s 2-tailed p-value also showed no bias for diabetes and hypertension studies (p=0.15 and p=0.13, respectively). For cardiovascular disease, visual inspection showed an under-representation of small studies.
Discussion
To our knowledge, this is the first systematic review and meta-analysis that explored the relationship between CMRF and the mortality and ICU admission rates in patients with COVID-19 in the Asia-Pacific region. Thirty-one high-quality studies were included, representing 84,011 patients for analysis, being the largest meta-analyses in this region to date.
There are four main findings from the evidence provided by this review. First, the pooled mortality rate was 9.3%, and pooled ICU admission rates were 9.6%. Second, diabetes, hypertension or cardiovascular disease were associated with a higher likelihood of mortality and ICU admission with COVID-19. Diabetes was the most salient predictor of mortality risk (2.85-fold increase), while patients with cardiovascular disease are at highest risk of ICU admission (3.32-fold increase). Third, our meta-regression analysis showed significant confounding by age, followed by sex and regional differences. Diabetes- and hypertension-increased mortality was attenuated in male and older patients, while cardiovascular disease-increased mortality was attenuated in older patients and those who resided in northern Asia (Supplementary Material Table 3). Fourth, obesity, smoking and hyperlipidaemia were not associated with mortality and ICU outcomes.
Our mortality estimate rates are consistent with prior reviews, with mortality estimates ranging from 9.9% to 13.9%.16,17 However, this is much higher than global estimates of 1.23%.1 This may be attributed to limited testing capabilities and challenges in attributing the confirmed causes of death to COVID-19.
Diabetes and Outcomes
In line with our findings, systematic reviews conducted on cohorts worldwide documented that diabetes is the most important cause of mortality in COVID-19-hospitalised patients.18 This is consistent in regions such as Europe, the US and Asia. Correlation strengths were also similar, with ORs ranging from 2.38 to 2.67.19–21 There are multiple mechanisms linking the severity of COVID-19 infection with diabetes. For instance, patients with diabetes have higher levels of expression of ACE2, the binding site for COVID-19, which facilitates infection and the risk of developing severe and fatal COVID-19.22 Diabetes may also lead to alterations in the immune response of individuals, leading to higher susceptibility.23
Interestingly, our observations suggest that the mortality risk associated with diabetes is significantly higher in younger compared with older COVID-19 patients, congruent with findings from several Western cohorts.24–26 Previous findings suggest that specifically, after the age of 50 years, diabetes-related mortality risk is attenuated by all other comorbidities associated with age.27 A nationwide cohort study in England found that the relative risk of in-hospital death for younger age groups with diabetes was significantly higher than in older age groups, although the absolute risk in lower age groups was small. This may be partly attributed to a disproportionate amount of deaths in older age groups occurring outside of the hospital setting, given their frail status and multiple comorbidities.28 Recent evidence suggests that patients over the age of 50 have reduced immunity, lower organ reserve and higher expressions of ACE2, all of which contribute to the development of severe COVID-19 disease apart from diabetes alone.29
Hypertension and Outcomes
We observed a moderate effect of hypertension on the risk of mortality, which was similar to previous studies, with pooled ORs ranging from 2.2 to 2.7.8,30,31 We also observed that hypertension was the most common comorbidity among patients with COVID-19. Like diabetes, the apparent adverse effect of hypertension was attenuated in analyses adjusted for age and sex. Despite age being an important source of confounding, several studies did not analyse the effect of age and sex as potential confounders, which may lead to erroneous conclusions about the risk factors having independent associations with mortality in patients with COVID-19. The effect of anti-hypertensive medications was not explored in this review, although we acknowledge its influence on mortality risk as demonstrated previously.32
Being a man also attenuated the effect of diabetes and hypertension on mortality. Multiple studies highlight higher fatality rates in men compared with women globally.7,33 The possible reasons are as follows: at the molecular level, women are proposed to have stronger immune systems leading to fasting recognition of viral components. Testosterone has also been shown to have a suppressive effect on the immune system.34 Several preclinical studies also propose that ACE2, the protein that facilitates SARS-COV-2 entry, is more frequently expressed in males compared to females, especially under pathological stress.35 Sex differences in risk behaviours, such as smoking and drinking being higher in men, may contribute to sex differences in lung damage.36
Cardiovascular Diseases and Outcomes
Unique to our study, we found that regional differences between North and South Asia significantly influenced the impact of cardiovascular diseases on mortality. Patients in northern Asia with cardiovascular diseases had a higher likelihood of mortality (OR 4.37) compared with patients in South Asia (OR 2.18). Countries in northern Asia had a greater proportion of high-middle income countries (HMIC), while the South Asian countries constituted more LMICs. Studies from the World Bank showed significantly higher all-cause mortality rates in patients with COVID-19 from LMICs compared with those from HMICs, which is in line with our findings.37 Possible explanations may include a higher number of elderly people in HMIC populations, as the disease targets the aged and those with comorbidities. Mortality rates are more likely to be correctly attributed when death occurs in a hospital, and in wealthy countries most deaths occur in hospitals or other institutions. This contrasts with LMICs where more deaths occur out of hospital and therefore may be under-attributed to COVID-19.37
Obesity, Smoking and Hyperlipidaemia
Despite obesity being a risk factor for development of other cardiometabolic risk factors, limited studies included anthropometric measurements to allow a better evaluation of the impact of obesity.38 Our study found that obesity was not associated with an increased risk of mortality or ICU admission rates. This could be limited by a relatively small sample size for obesity, leading to a Type 2 error. An umbrella review examining 27 systematic reviews found discordant results on the associations of obesity with COVID-19 mortality, although the data trended towards increased mortality.39 Similarly, we also found non-significant effects of smoking and hyperlipidaemia on mortality and ICU admission, which was consistent with other larger reviews.40,41
Uniquely, several countries within the Asia-Pacific region were substantially impacted by previous infectious respiratory diseases, leading to aggressive actions taken from the beginning of the pandemic and, hence, lower individual mortality rates compared to Western counterparts such as Italy. In this review, South Korea consistently demonstrated low rates of mortality and ICU admission rates in large database studies.14,15,42 Previous experience with Middle East Respiratory Syndrome in 2015 exposed the vulnerabilities of the South Korean healthcare system. Subsequently, more negative-pressure isolation rooms were built, enhanced testing capabilities with rigorous contact tracing enabled rapid detection of cases and treatment, and successful, effective health communications all contributed to the mitigation of the effects of COVID-19.42,43 Despite being at the epicentre of the pandemic, China’s overall case fatality rates remain low at the time of writing, concordant with several studies in this review.32,44,45 Lessons learned from the SARS epidemic in 2002–2004 led to improvements in risk communication, curtailing the movement of people, mass gathering restrictions, enhanced border security and increased contact tracing. Similar patterns were also observed in countries within the region such as Hong Kong, Taiwan and Singapore that were previously affected by SARS. In general, Asian countries demonstrated swifter and more aggressive approaches, while western countries – although having varying degrees of responsiveness – trended towards a mitigation approach and tended to be less stringent.46
Furthermore, it is interesting to observe intra-regional differences in the sex predominance of COVID-19 cases. Our results demonstrated a female predominance in South Korea and a male predominance in Abu Dhabi, Bangladesh, India, Iran and Pakistan.43,47–55 The first outbreak in South Korea was at a religious site in Daegu city, with predominantly female members. This suggests that external factors, such as religious attendance, can exert a huge influence on the sociodemographic presentation of COVID-19. Apart from biological and lifestyle differences, reasons for male predominance in the aforementioned countries may be attributed to greater number of male societal interactions, through professional or laborious work or religious gatherings. In Pakistan, most mosques are only for males, while nearly 99% of females conduct worship in their own homes, minimising transmission.56 Previous research also indicated sex disparities in access to healthcare services, where women were less likely to be admitted to hospital compared with men in India.57 As such, sex disparities observed from the COVID-19 pandemic may indicate underlying persisting systemic differences, which can be explored in future studies.
Essentially, this study provided an aggregate estimate of the potentiating effects of CMRF in COVID-19 patients. The qualitative effects and bearings are concordant with several other worldwide meta-analyses, although precise quantitative estimates should be interpreted with caution. Owing to the wide variability of healthcare systems, testing and case definitions of what constitutes a severe disease, the true magnitude of its impact may vary. The majority of studies were conducted prior to the release of vaccines being validated by the WHO for emergency use (Sinovac, China in June 2020, Pfizer and Moderna vaccines in December 2020). Therefore, although vaccination status could have been a potential confounder, the results in this study will not be affected, given that all studies were conducted prior to December 2020. The studies conducted in China were all conducted prior to June 2020, hence none of the patients would have had the opportunity to receive the Sinovac vaccines at that time.
Practically, knowledge of the sociodemographics and comorbidities that place COVID-19 patients at severe outcomes may aid clinicians in developing risk stratification workflows with the aim of prioritising such patients. This paper suggests that, beyond CMRF, age and sex are two of the most important influential factors on mortality in patients with COVID-19. This is not surprising and it is expected that age should have a significant impact on the course of the disease. Measures taken by public health policies to protect this vulnerable group, such as prioritising the elderly for vaccination and healthcare, were steps taken in the right direction. The health repercussions of COVID-19 aggregated in patients with CMRF veritably remind us of the cornerstone principles in public health prevention and regular management: optimisation of glycaemic outcomes, management of hypertensive profiles and adequate anti-thrombotic strategies to circumvent cardiovascular disease, especially in the elderly population.
Strengths and Limitations
The strengths of this study are in the large sample of patients, multivariate adjustments in age and sex, and evaluation of the impact of several CMRFs, including those that are less reported such as obesity and hyperlipidaemia. Study selection was also stringent in that only studies that had a low risk of bias, those that had adequate information, appropriate follow-up time and sufficient case numbers were included. The focused nature of this study on the Asia-Pacific region also facilitated intra-regional comparison.
Our study had several limitations. First, the true effect of CMRF in COVID-19 patients may have been attenuated by underestimation of COVID-19 mortality. Marked variation exists between countries in the healthcare systems and their response towards the crisis, testing availability and case definitions. Patients may have delayed seeking or avoided medical care, and deaths from being turned away by overwhelmed health systems and stretched capacities may also remain unaccounted for. Consistent underestimation of mortality by at least one-third may reflect the composite inadequacies despite exhaustive efforts to combat the crisis.58 However, the aim of this study was not to provide a specific point estimate of cardiovascular incidences but rather to examine the correlations in outcomes among COVID-19 patients with CMRF.
Second, studies that focused exclusively on ICU patients or patients with 100% mortality were excluded to minimise selection bias as these patients tend to have higher rates of mortality, ICU admission rates and prevalence of cardiovascular risk factors.
Third, our meta-analysis was limited by substantial heterogeneity, which was accounted for by performing univariate and multivariate meta-regression analysis on the most likely sources of potential confounders, including age, sex and regional subgroups. We recognise that residual confounding is likely to remain from other major comorbidities, such as high BMI, chronic obstructive pulmonary disease, kidney disease and other potential confounders. Heterogeneity may also result from structural factors, such as differences in testing capability, healthcare delivery and vaccination uptake rates.
Fourth, several studies in this review did not explicitly define what constitutes cardiovascular disease. This may pose a problem with interpretation as cardiovascular disease is a collective term that encompasses a wide range of vascular events. Classically, cardiovascular disease refers to four entities: coronary heart disease, cerebrovascular disease, peripheral artery disease (PAD) and aortic atherosclerosis.59 Papers reviewing the effects of cardiovascular disease on mortality should also include the different subtypes of cardiovascular disease in their study to allow more focused and direct comparisons. Several studies assessed cerebrovascular disease or stroke, PAD and heart failure separately from cardiovascular disease. Despite the limitations, a subgroup analysis was performed to exclude five studies that explicitly included cerebrovascular disease and heart failure and there was no significant difference in the results for mortality (Supplementary Material Table 5).
Fifth, several studies included both hospitalised and community patients with COVID-19 without providing a breakdown of the numbers for each category and for those who died or were admitted to ICU. This impacts the understanding of mortality and ICU rates as patients who presented in the outpatient setting without a transfer of care are less likely to progress to severe outcomes. The majority of studies (22 out of 31) included hospitalised patients only and there were at least 50,805 out of 84,011 hospitalised patients. Hence, the results are more generalisable to inpatients compared to community patients, although precise estimates may differ.
Finally, studies in this systematic review were selected based on relevance to study outcomes, low risk of bias and robustness of statistical analysis. It is incidental that an almost equal proportion of studies from both North and South Asia were represented and efforts were made to avoid possible selection bias. Many other studies from the Asia-Pacific region were excluded from the review, including several articles from the Oceania region (e.g. Australia, New Zealand) and Southeast Asia (Singapore, Malaysia), as these articles did not fulfil our review criteria. Countries without published data are also not included. Hence the results may not be generalisable to these other regions.
Conclusion
This systematic review and meta-analysis demonstrated the consequences of diabetes, hypertension and cardiovascular disease on mortality and ICU admission among patients with COVID-19 in the Asia-Pacific region. Moreover, age, sex and regional differences substantially influenced the impact on the outcomes of patients with CMRF and COVID-19.
Click here to view Supplementary Material.
Clinical Perspective
- Among selected studies in the Asia-Pacific Region, diabetes, hypertension and cardiovascular disease were found to be associated with increased mortality and intensive care unit admission rates.
- Hyperlipidaemia, obesity and a history of smoking were not associated with increased mortality and intensive care unit admission rates.
- Age, sex and regional differences were found to be significant confounders on the effects of cardiometabolic risk factors on mortality in this study.