Functional evaluation is crucial for diagnosing undetermined coronary artery stenosis in stable coronary artery disease.1–3 Fractional flow reserve (FFR) is the gold standard for assessing the physiological severity of coronary stenosis.4 Several trials have revealed a reverse correlation between post-interventional FFR and the risk of adverse events.5,6 Many studies endorse FFR-guided interventions and current guidelines recommend FFR assessment for obstructed/mediated ischaemia.7,8 However, FFR guidance has its limitations, such as the requirement for drug-induced hyperaemia (typically using adenosine), patient discomfort, prolonged procedure time and reimbursement challenges.
To address these limitations, various methods based on 3D imaging modalities have been developed to derive FFR without invasive pressure wires or hyperaemic agents. One such 3D imaging modality is quantitative flow ratio (QFR), a novel technique capable of rapidly computing FFR from coronary angiography.9 QFR demonstrates a strong correlation with FFR and has proven clinical value in guiding management both pre- and post-percutaneous coronary intervention (PCI).9,10
This pilot study aimed to evaluate the impact of QFR-guided interventions in influencing decision-making processes for angioplasty and post-intervention procedures within the cardiology unit at the University Malaya Medical Centre (UMMC).
Methods
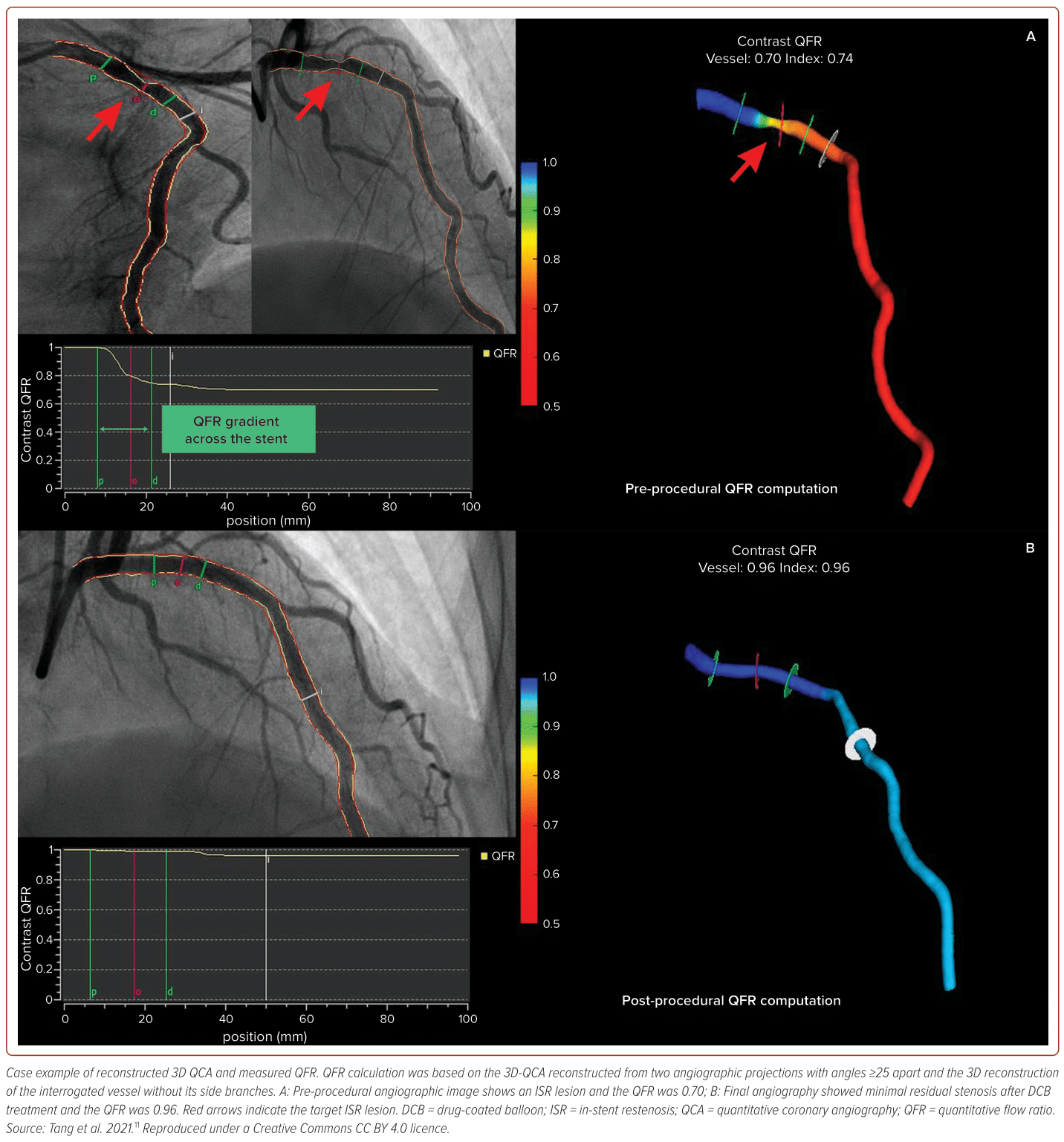
This pilot study was conducted at UMMC from 1 January 2023 to 31 July 2023 and involved all patients undergoing angiogram and angioplasty procedures. Patients were chosen based on specific inclusion and exclusion criteria (Table 1). Following the initial angiograms, the cardiologist assessed a vessel identified with borderline lesions in which the stenosis of the vessel was between 50% and 70% by using angiographic visual assessment, contemplating the choice between proceeding with PCI or opting for medical therapy. To enhance the decision-making process, QFR readings were conducted, serving as a pivotal tool to weigh up the need for intervention. QFR analyses were conducted both pre- and post-angioplasty using an advanced offline computerised system. Essential parameters, such as the reference vessel diameter, minimal lumen diameter and percent diameter stenosis, were meticulously measured and analysed (Figure 1).11 A QFR value >0.80 was considered non-significant, while a value <0.80 indicated significant stenosis, prompting the decision to continue with angioplasty. Post-angioplasty, the cardiologist reassessed the intervention’s success to determine whether further action was warranted. The lesion underwent re-evaluation using QFR and if the QFR surpassed 0.90, it signified a successful intervention with no need for additional measures. This approach ensured a thorough evaluation and an informed decision-making process when managing coronary lesions.
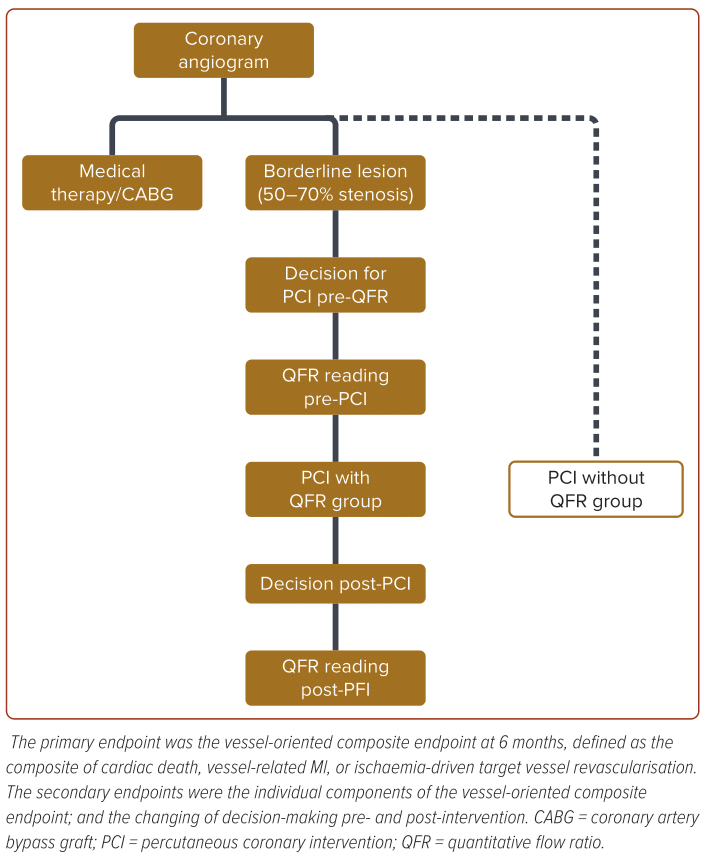
The primary objective of the study was to collect and analyse data related to the decision-making process before and after QFR readings and post-angioplasty assessments. Outcomes were assessed at discharge and during a 6-month follow-up for both the PCI with QFR group and the PCI without QFR group (Figure 2).
Statistical analysis involved presenting continuous variables as medians with interquartile ranges. Group comparisons for PCI with QFR and PCI without QFR was conducted using the Mann–Whitney U-test for continuous variables. Categorical variables will be summarised using frequencies and proportions and comparisons made using Pearson’s χ2 or Fisher’s exact test. The study received ethical approval from the UMMC Medical Research Ethics Committee (MREC).
Results
Of the 770 coronary angiograms performed during the study’s timeline, 122 patients exhibited borderline lesions (50–70% stenosis), 122 had significant stenosis (>70%), and the remaining 526 displayed normal or mild stenosis. Subjects with borderline lesions underwent QFR measurement, while those with significant stenosis underwent PCI without QFR measurement, and those with normal or mild stenosis received medical therapy. Subjects were divided into two groups: PCI without QFR (n=122) and PCI with QFR group (n=122).
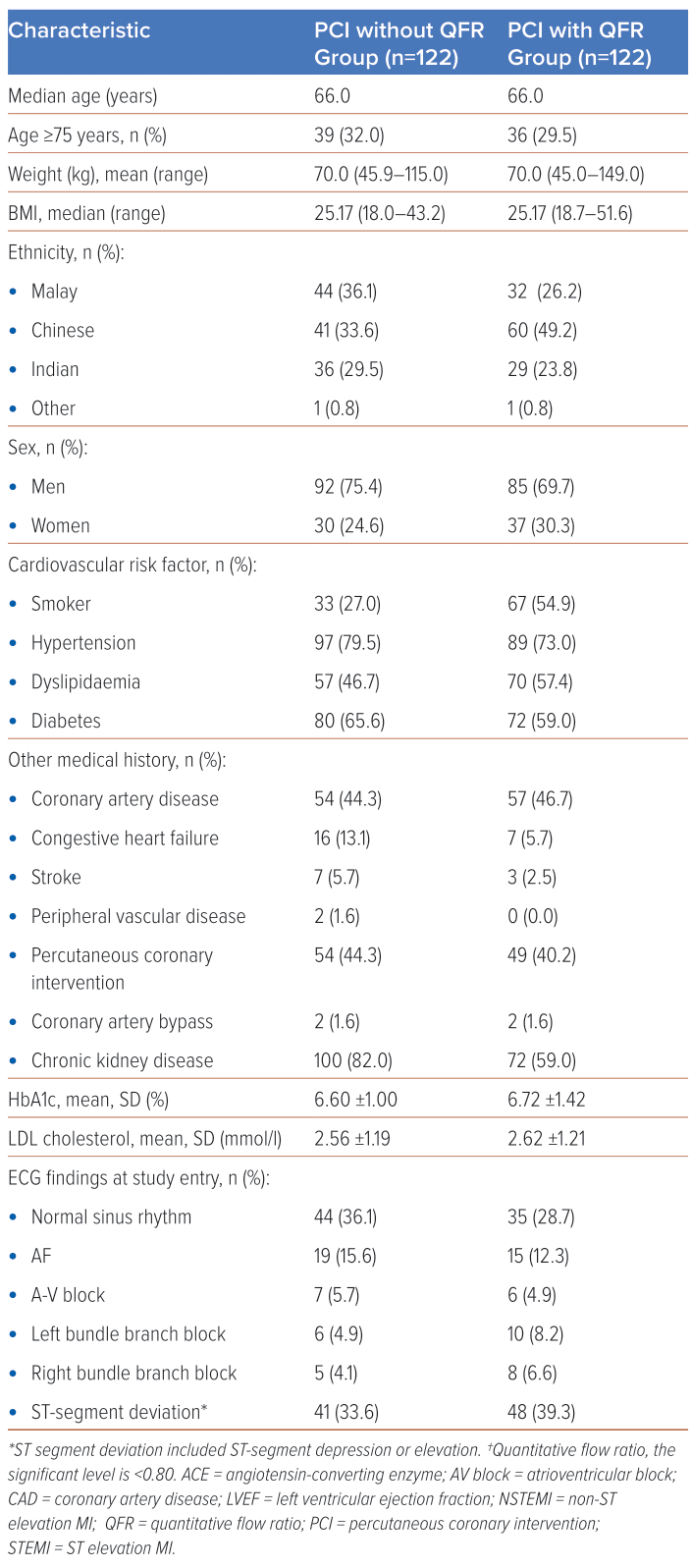
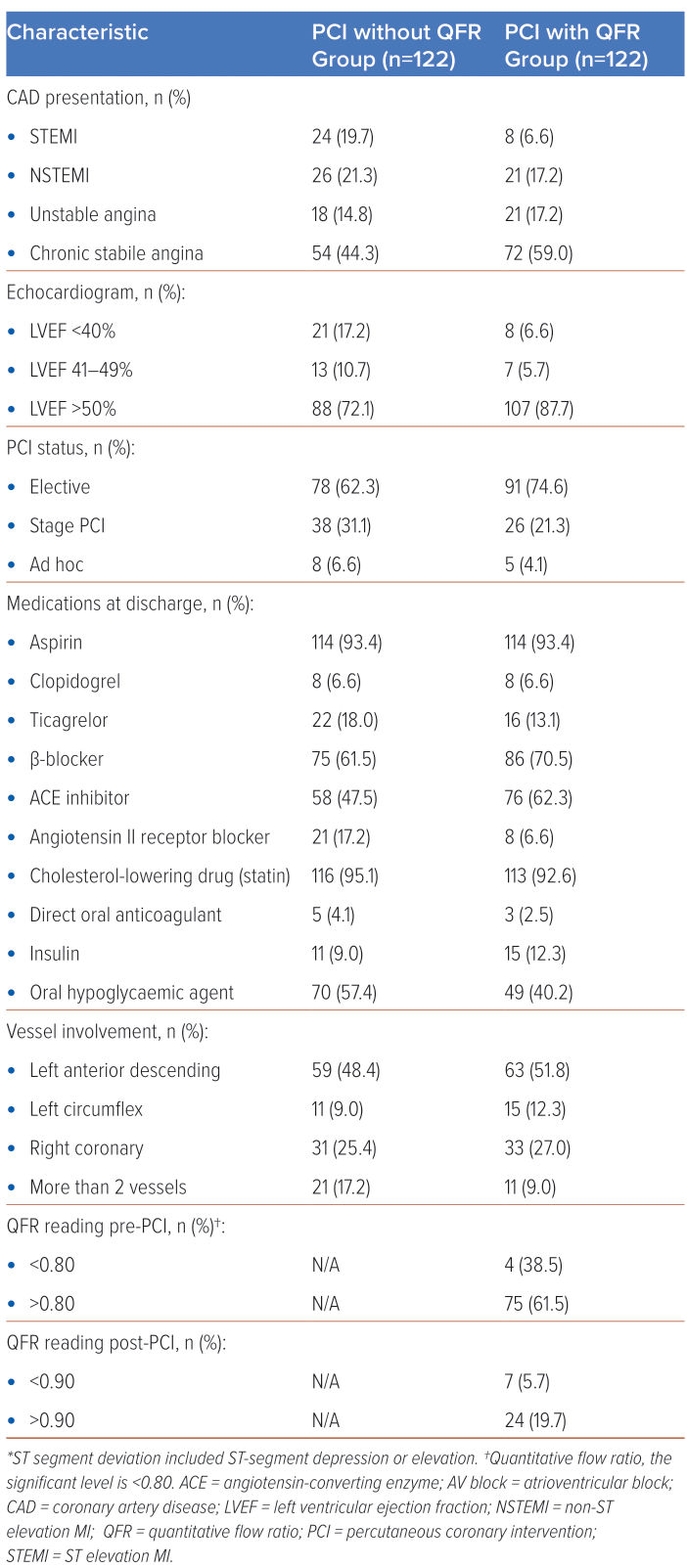
The baseline characteristics of the patients according to the treatment group are shown in Table 2. The median age for both groups was 66 years. Patients aged ≥75 years constituted 39 (32%) versus 36 (29.5%) for the PCI without QFR and the PCI with QFR, respectively. The mean body weight for both groups was 70 kg and the median BMI was 25.17 kg/m2. The PCI without QFR group was predominantly made up of Malaysian patients (44, 36.1%), while the PCI with QFR group had more Chinese patients (60, 49.2%). There were more men than women in both groups (92, 75.4% versus 85, 69.7%). More than half of the study population were non-smokers, with 33 (27%) in PCI without QFR and 67 (54.9%) in PCI with QFR being habitual smokers. For both groups, hypertension was the most common high-risk factor for cardiovascular disease, followed by diabetes and dyslipidaemia. Most patients also had chronic kidney disease. Glucose control was good in both groups and the mean LDL cholesterol (LDL-C) was 2.56 mmol/l ±1.19 versus 2.62 mmol/l ± 1.21, respectively. The majority exhibited a normal sinus rhythm in ECG (44, 36.1% versus 35, 28.7%), followed by ST-segment deviation (41, 44.3% versus 48, 39.3%). Chronic stable angina (54, 44.3% versus 72, 59.0%) was the most common coronary artery disease presentation, with preserved ejection fraction >50% in 88, 72.1% versus 107, 87.7%, respectively. Most procedures were elective. Aspirin and statins were the most frequently prescribed medications at discharge. The left anterior descending artery (LAD) was the most frequently involved vessel in both groups. The normal QFR reading (>0.80) pre-PCI was found in 75 patients (61.5%), and 24 (19.7%) had a normal QFR reading post-PCI (Table 2).
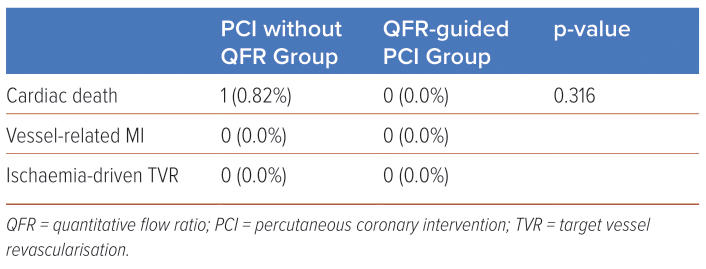
The results for the primary objective of the VOCE at 6 months follow-up showed one cardiac death in the PCI without QFR group and none in the PCI with QFR group, with no statistical significance (p=0.316) (Table 3). The secondary endpoints examining individual components of VOCE showed only one case of cardiac death in the PCI without QFR group. Another secondary endpoint, the changing of decision-making, indicated more changes in pre-intervention assessments rather than post-interventional assessments.
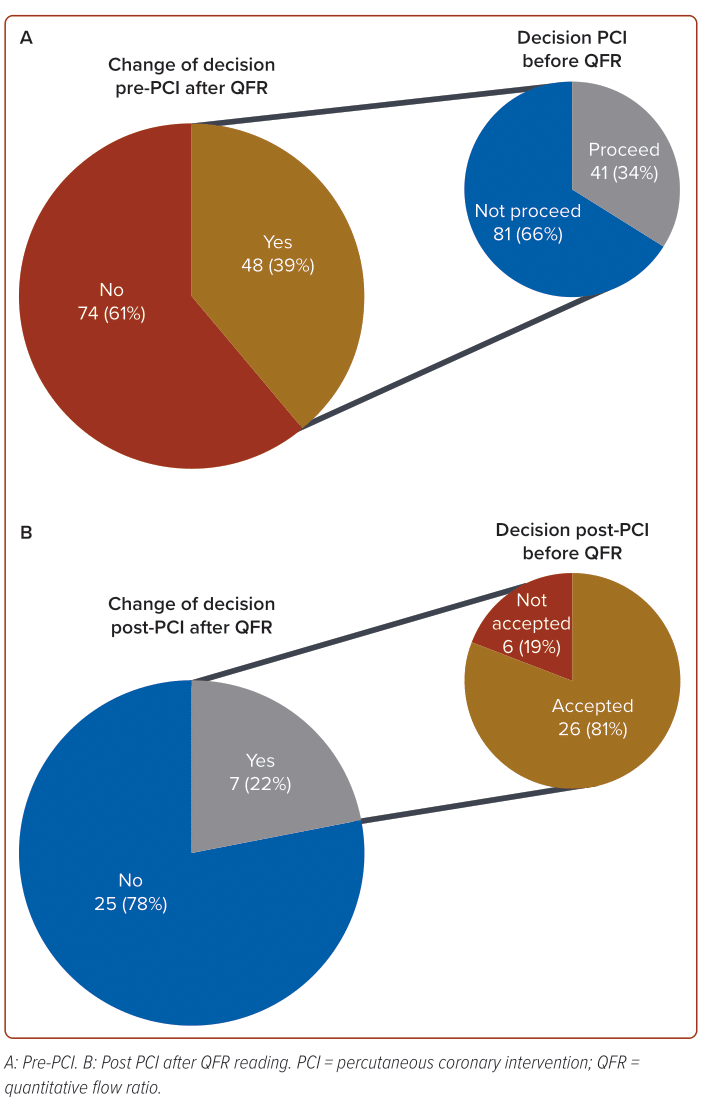
Among the 122 subjects who underwent QFR measurement, 48 individuals (39%) changed their decision following the QFR assessment (Figure 3). Prior to the QFR reading, the initial decision was to proceed with angioplasty in 41 cases (34%) and not to proceed in 81 cases (66%). In the group initially slated for angioplasty (n=41), QFR readings revealed that 19 individuals (46%) had a QFR >0.80, indicating a non-significant condition, while 22 patients (54%) had a QFR <0.80, signalling a significant stenosis requiring intervention. On the other hand, among the 81 subjects initially earmarked not to proceed, QFR readings demonstrated that 55 patients (68%) had a QFR >0.80, signifying a non-significant condition, while 26 patients (32%) had a QFR <0.80, suggesting a significant stenosis despite the initial decision not to proceed with the intervention.
In evaluating the impact of QFR readings on decision-making post-PCI, it is noteworthy that only a minority – specifically 7/32 cases (22%) – experienced a change in decision. Among these cases, 6/32 (19%) initially rejected the post-PCI results and sought further intervention, while the majority, constituting 26/32 (81%), accepted the results without the need for additional measures. In the group that accepted the post-PCI results (26/32), subsequent QFR readings revealed that 19 patients (73%) had a QFR >0.90, which confirmed the results, while the remaining 7 patients (27%) had a QFR <0.90, suggesting that the initial decision should be reconsidered. Conversely, in the group that initially rejected the post-PCI results (6/32), QFR readings showed that only one (17%) had a QFR <0.90, indicating a persistent non-acceptance, while the remaining five patients (83%) had a QFR >0.90, prompting a reversal of the initial decision. Highlighting the notably modest occurrence rate of alterations to initial decisions and underscoring the diverse outcomes contingent upon the acceptance or dismissal of the initial post-PCI findings. These results also show that QFR readings are helpful for decision-making about borderline lesions before PCI. The visual assessment for post-PCI angiographic results in our study revealed a similarity with post-PCI QFR reading, where the standard value of the QFR post-PCI being >0.90.
Discussion
The integration of functional evaluation in stable coronary artery disease has become pivotal, especially in cases of undetermined coronary artery stenosis. FFR has long been established as the gold standard for assessing the physiological severity of coronary stenosis.12 This diagnostic tool has gained prominence due to its ability to provide crucial insights into the functional significance of lesions, guiding therapeutic decisions and affecting clinical outcomes.1–3 Numerous trials have consistently demonstrated a reverse correlation between post-interventional FFR and the risk of adverse events.5,6,12–16 The endorsement of FFR-guided interventions in many studies underscores their clinical usefulness in guiding coronary revascularisation. Current guidelines further support FFR assessment for ischaemia caused by obstructed coronary arteries, reinforcing its status as a class IIa recommendation.7,8
Despite their recognised benefits, FFR-guided interventions have intrinsic limitations. The necessity for drug-induced hyperaemia, often achieved through adenosine administration, poses challenges. Patient discomfort, prolonged procedural times and issues related to reimbursement further contribute to the limitations associated with FFR-based diagnostic strategies.9 In response to these challenges, innovative approaches using 3D imaging modalities have emerged. QFR stands out as a notable technique, offering a non-invasive and efficient alternative to traditional FFR measurement.17,18 By rapidly computing FFR from coronary angiography without the need for pressure wires or hyperaemic agents, QFR has demonstrated strong correlations with FFR, establishing its clinical value in guiding both pre- and post-PCI management.19–21
The current pilot study conducted at the University Malaya Medical Centre sought to assess the impact of QFR-guided interventions on decision-making processes related to angioplasty and post-intervention procedures. The study cohort exhibited a diverse range of coronary artery conditions. Notably, subjects with borderline lesions, significant stenosis and normal or mild stenosis were systematically managed based on QFR guidance, PCI without QFR and medical therapy, respectively. The baseline characteristics of the study population (Table 2), highlight the demographic and clinical diversity within the PCI without QFR and PCI with QFR groups. These characteristics, including age, weight, BMI, ethnicity and comorbidities, provide a comprehensive overview of the patient profile.
The primary endpoint analysis of the VOCE at 6 months revealed insights into the safety and feasibility of QFR-guided interventions. While the observed difference in cardiac death rates between the PCI without QFR and PCI with QFR groups did not reach statistical significance (p=0.316), it is noteworthy that the absence of cardiac deaths in the QFR-guided group underscores the potential benefits of QFR in improving patient outcomes. A comparative perspective is offered by the 2023 study conducted by Barauskas et al., which demonstrated a significant reduction in mortality and revascularisation at 12-month follow-up and an improvement in the quality of life of patients with ST-elevation MI when QFR was employed in non-culprit lesions.22 This reinforces the growing body of evidence supporting the favourable impact of QFR on clinical outcomes. Similarly, the study by Song et al. in 2022 aligns with our findings, indicating that QFR-guided lesion selection resulted in improved 2-year clinical outcomes compared to standard angiography guidance.23 These studies collectively strengthen the argument for the positive influence of QFR on long-term clinical endpoints, extending beyond the scope of our current investigation. The differing timelines of follow-up in these studies (12-month and 2-year follow-ups) contribute valuable insights into the sustained benefits of QFR over an extended period.
While the primary endpoint sheds light on the overall safety of QFR-guided interventions, the secondary endpoints examining individual components of VOCE provide a more nuanced understanding. With only one instance of cardiac death identified in the PCI without QFR group, it is evident that QFR may contribute to a more favourable clinical profile. One of the observations in our study is the substantial impact on the decision-making processes, particularly in the pre-intervention phase. Decision changes occurred in 39% of subjects who underwent QFR measurement, highlighting the clinical relevance of QFR in guiding treatment strategies. This finding resonates with the study by Lee et al., which demonstrated the good diagnostic performance of resting QFR (rQFR) in predicting residual ischaemia after PCI (AUC: 0.856 [0.804–0.909]; p<0.001).24 Furthermore, rQFR was effective in predicting the incidence of 2-year outcomes after index PCI, showing comparable performance to post-PCI FFR (AUC: 0.712 [0.555–0.869]; p=0.041).24 These findings suggest that QFR, both in its resting state and post-PCI assessment, has a robust predictive capacity, enhancing its role in decision-making processes.
The post-PCI decision-making changes following the QFR reading were relatively modest, with 22% experiencing shifts in decisions. Crucially, the majority (81%) accepted the QFR results, underscoring the potential trust and confidence in QFR assessments even after PCI. This aligns with the broader perspective that QFR, as demonstrated by Lee et al., remains effective in predicting outcomes post-PCI, comparable to actual post-PCI FFR.24 The findings suggest that QFR readings are crucial in decision-making processes, particularly in the pre-PCI phase for borderline lesions. While post-PCI assessments with QFR may not significantly alter decisions compared to visual assessments, the study indicates that both approaches yield comparable results.
The implications of our study extend to offering a valuable reference for the integration of QFR assessment within the field of interventional cardiology, particularly in clinical practice. Our findings carry significance for guiding decision-making in both pre- and post-angioplasty procedures, providing a foundation for future research directions in this domain. The insights gleaned from this study could potentially influence and shape the evolving landscape of interventions in cardiology, enhancing the precision and effectiveness of decision-making processes in clinical settings.
Study Limitations
Our study has a few limitations. The single-centre pilot study was conducted at the University Malaya Medical Centre and its findings may not be universally applicable. Consequently, the generalisability of our findings may be restricted, given the potential variability in demographic and clinical characteristics across a more diverse patient population. To address this limitation and enhance the robustness of our conclusions, future research must encompass multicentre studies involving a broader spectrum of healthcare settings and patient profiles.
While the sample size of 770 subjects is considerable, it may be insufficient to capture the full spectrum of clinical scenarios and potential outcomes. Larger multicentre studies are needed to enhance the robustness and generalisability of findings. It also had a short follow-up duration of 6 months and although this allows for valuable insights, it may not fully elucidate the long-term implications of QFR-guided interventions. The evolving nature of coronary artery disease warrants more extended follow-up periods to assess sustained benefits and potential late complications.
The study population was divided into distinct groups based on lesion severity and the decision for QFR-guided interventions, PCI without QFR, or medical therapy. This approach may introduce selection bias, limiting the generalisability of findings to a more diverse patient population. The study employed predetermined treatment strategies based on QFR guidance, which may not reflect real-world scenarios where clinical decision-making is more nuanced. The rigid assignment of interventions could influence the interpretation of results and limit the exploration of alternative management approaches.
The study includes a predominantly Asian population from a specific geographic region (Malaysia). The ethnic and regional homogeneity may limit the generalisability of findings to more ethnically diverse populations in other parts of the world. There was also an absence of randomisation in treatment allocation which may introduce biases in patient selection and confounding variables, potentially affecting the internal validity of the study. Last, while the study provides valuable insights into secondary endpoints, such as decision-making processes, it does not delve into the qualitative aspects of the changes in decisions that were made. Understanding the factors influencing shifts in decisions could aid the assessment of the clinical impact of QFR.
Conclusion
This study underscores the pivotal role of QFR measurements in the assessment of patients presenting with borderline angiographic lesions (50–70% stenosis). QFR has been shown to be a valuable modality, providing a nuanced understanding of coronary physiology and facilitating informed decisions regarding angioplasty. Our findings highlight the substantial benefits of QFR in improving decision-making in both pre- and post-PCI interventions. Notably, the incorporation of QFR measurements post-PCI significantly enhances our confidence when concluding the procedure, offering a more comprehensive evaluation of its success. While our results are promising and contribute valuable insights, it is crucial to acknowledge the need for further research. Future investigations should encompass larger and more diverse datasets, allowing for extended follow-up periods to comprehensively assess the impact of QFR on the VOCE. This ongoing research will play a pivotal role in solidifying the significance of QFR in clinical practice and unveiling its potential benefits for patients with borderline angiographic lesions. 
Clinical Perspective
- This study offers a valuable reference for integrating quantitative flow ratio (QFR) assessment in interventional cardiology.
- QFR is effective in guiding decision-making both before and after angioplasty procedures.
- Insights could shape the evolving landscape of interventions in cardiology, enhancing precision and effectiveness in clinical settings.