Tricuspid regurgitation (TR) is a common disease, and the tricuspid valve (TV) is no longer a ‘forgotten valve’. Open heart surgery for isolated TR is uncommonly performed due to high operative risk (8–10% mortality).1,2 However, TR is associated with increased morbidity and mortality.3 There exists an unmet clinical need for less invasive interventions to treat TR. A wide variety of technologies has been developed in recent years, among which, transcatheter edge-to-edge repair (TEER) of the TV is the most mature and commonly used technique to treat TR.4 In this review, we aim to discuss the clinical data, imaging and interventional techniques of tricuspid TEER.
Clinical Data
TEER is an established technique for treating mitral regurgitation. This technique is used to treat TR using the MitraClip device (Abbott Vascular) on off-label compassionate grounds.5 Retrospective registries suggested that this technique is safe and effective in TR reduction, and is associated with significant symptom improvement.6,7
Recently, a dedicated TEER system (TriClip; Abbott Vascular) with a shorter curve guiding catheter and additional steerable plane of motion (septal–lateral plane) was developed to facilitate coaxial TV access. The core laboratory adjudicated, multicentre prospective TRILUMINATE registry showed that TriClip is associated with a significant and durable reduction in TR (to moderate in 71%), and is accompanied by significant symptom improvement (83% remain at New York Heart Association functional class I/II) and a 40% reduction in re-hospitalisations at 1 year.8 Of note, patients with severe pulmonary hypertension (>60 mmHg), previous tricuspid valve procedures, a coaptation gap >10 mm or device leads that would prevent appropriate placement of a clip were excluded. Among the 85 subjects included, most of the TR treated was functional (84%), and AF (92%) was the most common comorbidity. Importantly, the reported major adverse events through 1 year and all-cause mortality were both only 7.1%, which compare favourably with real-world data on transcatheter tricuspid valve interventions (all-cause mortality 23%).9 The safety profile further made TEER an appealing treatment option for patients with symptomatic TR. Moreover, significant reverse right atrial and right ventricular remodelling were observed in terms of size and function.
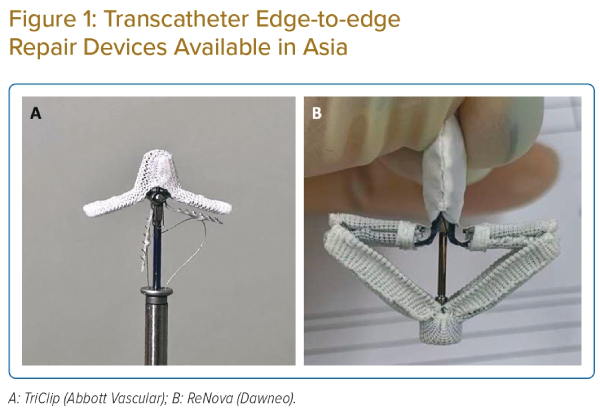
PASCAL (Edwards Lifesciences) is another TEER system with increasing clinical data on the treatment of TR in the US and Europe.10 In the multicentre CLASP-TR registry, an 88% procedural success, defined as implantation with at least more than one grade reduction in TR at its completion, was reported. A similar TR reduction rate (86% reduced to moderate or less) and reverse right atrial and right ventricular remodelling were reported preliminary at 1 year. A highly similar exclusion criteria were adopted in this registry including patients with severe pulmonary hypertension (>60 mmHg), previous tricuspid valve procedures, a coaptation gap >10 mm or a leaflet length <8 mm, or device leads that would prevent appropriate placement of a clip. In Asia, the ReNova TEER system (Dawneo Medical Technology) is under clinical development for the treatment of TR (Figure 1).
Anatomy of Tricuspid Valve and Imaging Evaluation
The TV is the largest cardiac valve, and a normal TV area is between 7 and 9 cm2.11 There are significant variations in the TV anatomy among individuals. However, in general, the anterior leaflet is the largest, while the semicircular septal leaflet has the smallest radial length. Characteristically, the septal leaflet is apically inserted relative to the septal insertion of the anterior mitral leaflet. The posterior leaflet has multiple scallops and the smallest annular circumference. The anterior papillary muscle is vital, as it supplies chordae to the anterior and posterior leaflets. The posterior papillary muscle provides chordae to the posterior and septal leaflets, and the septal papillary muscle may be absent in almost 20% of patients. In addition, the tricuspid annulus is a complex 3D structure, approximately 20% larger and less symmetrical than the ‘saddle-shaped’ mitral annulus.12 The greatest increase in cardiac output may be obtained with septal–anterior and septal–posterior leaflet tip clips. If a multiclip approach is used, closing the septal–anterior coaptation may yield the best result.
With increasing interest and need for TV intervention, echocardiography (transthoracic and transoesophageal) plays an increasingly important role in the evaluation of TR. In every TR case, it is fundamental to assess its severity, the TV leaflet morphology, the mechanism of TR and also the feasibility of TEER if intervention is contemplated. A new TR grading scheme, which classified TR into mild, moderate, severe, massive and torrential, has gained increasing clinical adoption.13 For TV leaflet morphology, Hahn et al. recently proposed a classification of the TV into six types/subtypes: type I, three leaflets; type II, two leaflets; type IIIA, four leaflets with two anterior; type IIIB, four leaflets with two posterior; type IIIC, four leaflets with two septal; and type IV, more than four leaflets.14
Next, it is crucial to understand the aetiologies of TR during imaging evaluation. Causes of TR can be classified into primary (intrinsic leaflet pathology), secondary or cardiac implantable electronic device (CIED)-related.15 Primary TR is caused by an abnormality of any of the components of the TV apparatus (tricuspid leaflets, chordae, papillary muscles or annulus) due to congenital or acquired causes.16 Secondary TR is the most common cause, and can further be classified into atrial functional or ventricular functional.4 In atrial functional TR, the patient usually has underlying AF, which results in severe right atrial enlargement and significant isolated annular dilatation with minimal leaflet tethering. In ventricular functional TR, there is pulmonary hypertension and right ventricular enlargement, leading to significant leaflet tethering and annular dilation. Moreover, assessment of right ventricular function and pulmonary pressure is also important for patient selection. In general, if the leaflet coaptation gap is >10 mm or systolic pulmonary artery pressure >60 mmHg, TEER is contraindicated.
CIED-related TR is complex, and is rarely purely due to mechanical interaction of the CIED lead and the TV (i.e. TV leaflet impingement, leaflet or chordal entanglement, chordal rupture, leaflet adherence or leaflet laceration or perforation), but secondary to pacing-related TR.17 TEER is unfavourable in CIED-related TR, but has been reported with a reasonable success rate in highly selected anatomy (e.g. lead distant from grasping zone, lead free of adhesion from adjacent leaflets).17 Furthermore, leaflet grasping could be challenging in the case of severe leaflet tethering.
Imaging Predictors of Tricuspid Regurgitation Reduction
A number of imaging predictors of significant TR reductions by TEER have recently been identified. The earliest and most important predictor of good TR reduction identified is the coaptation gap. Besler et al. found that a small coaptation gap size (<7.2 mm) and a central or anteroseptal TR jet location independently predicted the procedural success (TR reduction by ≥1 grade) of TEER using the MitraClip device.18
Ruf et al. later found that a coaptation gap ≤8.4 mm was predictive of a reduction to moderate or less TR, and a coaptation gap ≤10 mm is associated with both improvement in New York Heart Association class and improvement in 6-minute walk distance, but not in those with a coaptation gap >10 mm, after TEER using the MitraClip XTR device.19 Additionally, Sugiura et al. found that the four-leaflet TV configuration was associated with an increased risk of residual TR equal to or greater than severe after TEER, independent of baseline TR grade, coaptation gap width and TR jet location.20
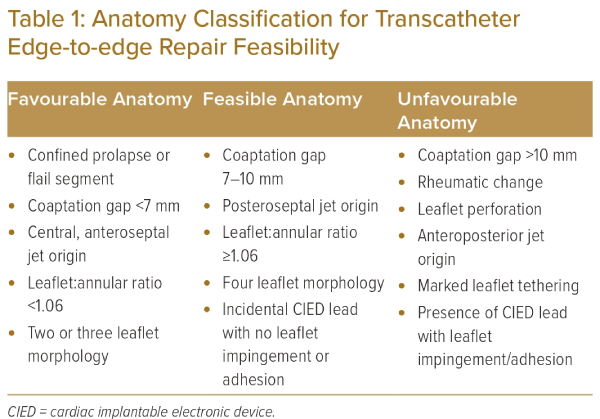
More recently, Tanaka et al. identified low leaflet-to-annulus index (<1.06, anterior + septal leaflet length/septal–lateral tricuspid annulus diameter) as a significant predictor of residual severe TR after TEER. With the current understanding and clinical experience, the TV anatomy can be classified into the favourable, feasible or unfavourable category for TEER (Table 1).
Favourable anatomy includes those with confined prolapse/flail segment, a coaptation gap of <7 mm, central or anteroseptal jet origin, a leaflet:annular ratio of ≥1.06, and two- or three- leaflet morphology. Feasible anatomy includes those with a coaptation gap of 7–10 mm, posteroseptal jet origin, a leaflet:annular ratio of <1.06, four-leaflet morphology or presence of incidental CIED lead with no leaflet impingement or adhesion. Whereas unfavourable anatomy includes a coaptation gap >10 mm, anteroposterior jet origin, rheumatic tricuspid leaflet change (leaflet retraction, calcification or subvalvular apparatus calcification), leaflet perforation, marked leaflet tethering or presence of CIED lead with leaflet impingement/adhesion.
Impact of Pulmonary Hypertension and Right Ventricular Reserve
Apart from anatomical consideration, in evaluating TR interventions, it is also important to consider the pulmonary pressure and right ventricular reserve. In patients with persistent high pulmonary arterial systolic pressure (PASP; >60 mmHg) by echocardiogram despite medical optimisation and diuretics, TEER is contraindicated. In patients that have elevated PASP, but <60 mmHg, we recommend a comprehensive right heart catheterisation to assess the severity and cause of pulmonary hypertension, to aid the decision on tricuspid valve intervention.
It was found that an invasive PASP ≥50 mmHg and a mean pulmonary artery pressure (mPAP) ≥30 mm were associated with increased mortality after TEER for TR (HR 4.3).21 In the same study, TR patients were classified into three groups: no pulmonary hypertension (mPAP <30 mmHg), predominantly post-capillary pulmonary hypertension (mPAP >30 mmHg, transpulmonary pressure gradient >17 mmHg) and predominantly precapillary pulmonary hypertension (mPAP >30 mmHg, transpulmonary pressure gradient <17 mmHg). It was shown that patients without pulmonary hypertension were at low risk for mortality (estimated 1-year survival rate 92%), whereas those with postcapillary pulmonary hypertension were at a slightly higher mortality risk (estimated 1-year survival rate 78%), and patients with precapillary pulmonary hypertension were at high risk for mortality (estimated 1-year survival rate 38%). Therefore, TEER might not benefit patients with TR and predominantly precapillary pulmonary hypertension.
Apart from PASP, right ventricular contractile response to increased afterload is another important prognostic marker in TEER. Brener et al. found that adequate right ventricular contractile response to increased afterload (TAPSE/PASP ratio >0.406 mm/mmHg) was associated with a decreased risk of all-cause mortality after TEER for TR (HR 0.57; p=0.023). This ratio could potentially inform patient selection and prognostication following TEER for TR.
Procedure-in-brief and Technical Considerations
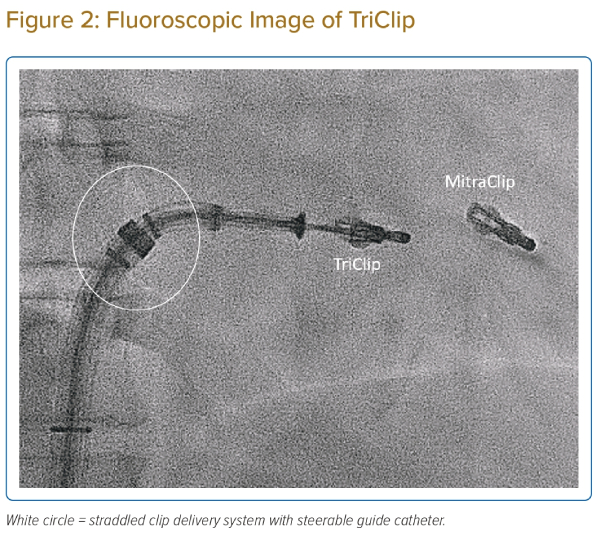
The TEER procedure was performed via femoral venous access under fluoroscopy and transoesophageal echocardiography (TOE) guidance, and mostly under general anaesthesia. First, the steerable guide catheter is inserted into the mid right atrium after serial skin dilatation. Second, the clip delivery system is inserted into the guide catheter and straddled (Figure 2). Third, the system is flexed into the TV plane. Fourth, the clip is opened, the trajectory of the system is optimised and the clip arm orientation is aligned perpendicular to the line of coaptation of the target leaflets at the right atrium. Fifth, the clip is then delivered into the right ventricle, and pulled back to grasp the target leaflets. Finally, after verification of the optimal target location and leaflet insertion, the clip is closed and then released.
TEER for TR was at first performed using the MitraClip device. When using the MitraClip device to treat TR, the ‘miskey’ technique was commonly adopted.22 The ‘miskey’ technique means that the operator inserts the clip delivery system with the blue line rotated 90° counterclockwise (miskey) and exits the steerable guide catheter straddled. This allows more versatile knob movements of the MitraClip system. After miskey, the ‘A’ knob of the MitraClip will function similar to the ‘M’ knob in mitral valve TEER, which allows navigation and entry into the TV. A frequently encountered issue of this off-label use is the inability to achieve a clip delivery system trajectory perpendicular to the TV annular plane due to the proximity of the clip delivery system to the interatrial septum (‘septal hugger’). Using left femoral venous access instead of right femoral venous access was described as a manoeuvre to reduce ‘septal hugger’.23
The new TriClip system (Abbott) is currently the only approved device for tricuspid TEER in the Asia-Pacific region. With the TriClip system, miskey is no longer necessary. The steerable guide catheter is made shorter for TV intervention. In addition, the knobs are simplified into F/E to allow flex and extend the delivery catheter to steer down to the valve plane, +/– knob to straighten and curve the guide for height adjustment above the valve, and the S/l knob to steer in the septal–lateral direction. The S/l knob could potentially help to optimise the alignment of the TriClip to the TV plane, correcting the trajectory from septal hugger and, hence, optimise the leaflet insertion and TR reduction.
Early experience of the tricuspid TEER procedure identified more single leaflet device attachment in comparison with mitral procedures (~7% in the TRILUMINATE study). This might be related to the suboptimal alignment of the clip to the TV plane with off-label MitraClip use, the difficulty in TV imaging to ensure adequate leaflet insertion and also the presence of a significant coaptation gap.24 Apart from the S/l knob modification, the latest TriClip Generation 4 offers four different implant sizes and controlled gripper actuation to permit optimised independent leaflet grasping. This could potentially expand the use of TriClip in treating TR with a larger coaptation gap (7–10 mm).
Apart from the device modification, a few techniques were described to reduce the coaptation gap and facilitate successful leaflet grasping. First, preoperative diuresis is important, which might reduce the tricuspid annulus dimension and, hence, reduce leaflet coaptation gap. Second, anterior or right-sided chest wall compression has also been described to reduce the TV annulus dimension and coaptation gap.25 Third, a standardised tilting of the intervention table by 10° (feet low) was also described to reduce the coaptation gap.26 If all the aforementioned fail, the ‘zipping’ technique can be considered, in which multiple clips are used, with the first one placed closed to the commissure, to facilitate subsequent clipping.5
Intraprocedural Imaging Guidance
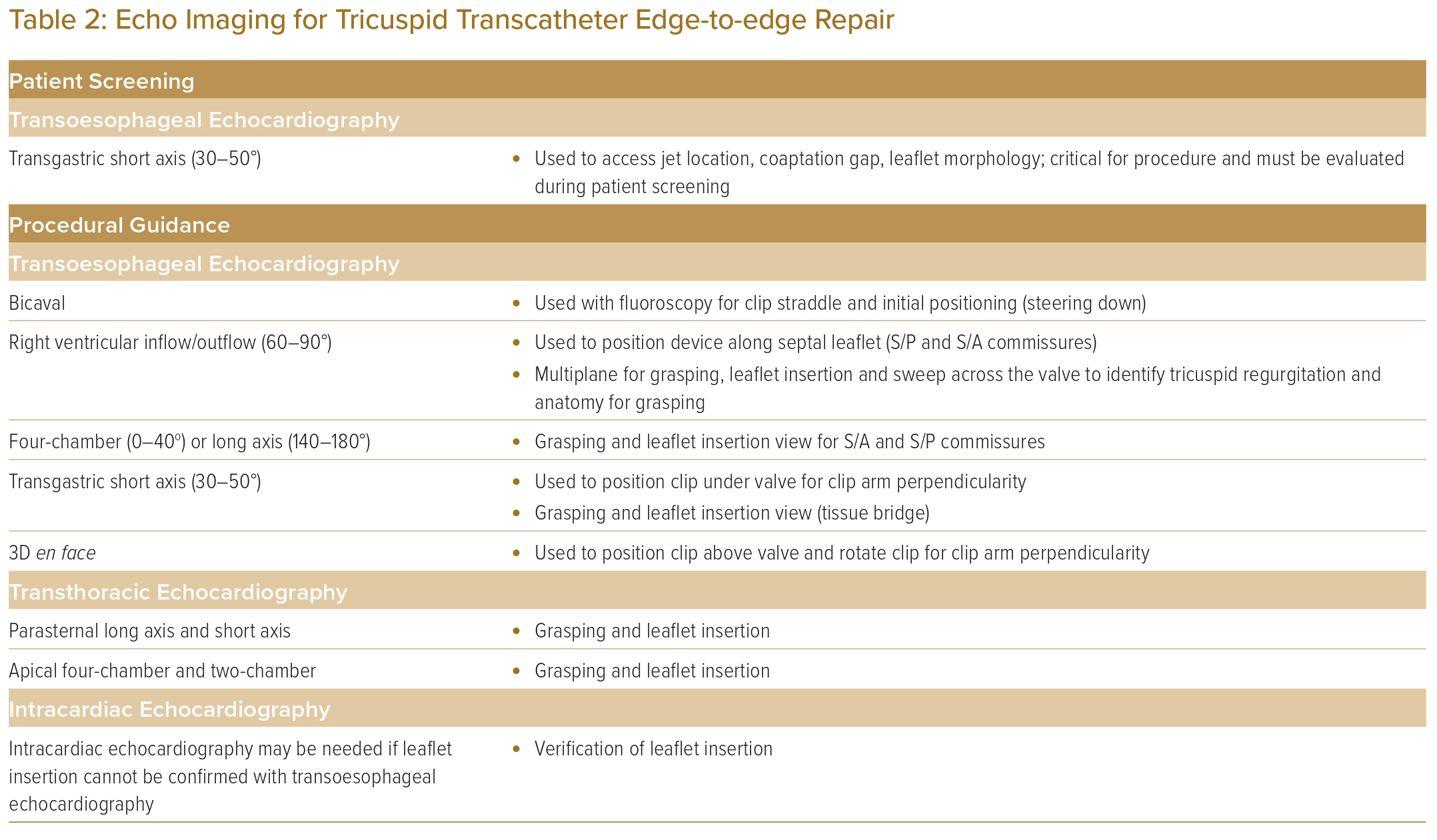
In performing TEER for TR, imaging guidance is of the utmost importance. 3D TOE remains the most commonly used imaging modality to guide TEER, in addition to standard fluoroscopy. TOE guides the navigation of the clip delivery system, tricuspid leaflet grasping and also assesses the TR reduction.11,27 Clear and continuous communication between the interventional imager and interventionist is the key to procedural success. TOE views and manoeuvres to obtain them are presented in Table 2.
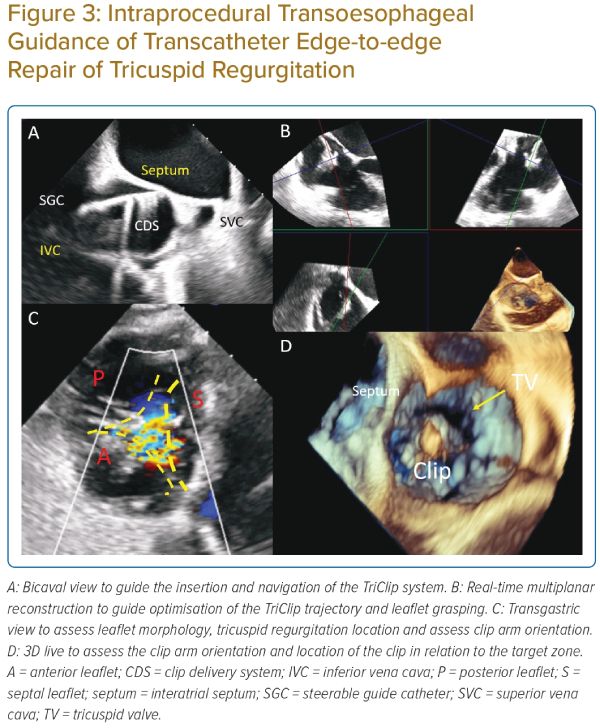
The bicaval or modified bicaval view (~80–110°) is used to guide the introduction of the guide and clip delivery system into the right atrium, with particular attention to avoid injury to the atrial septum (Figure 3A).28
The midoesophageal right ventricular inflow–outflow (‘commissural’) view at ~60° is used to direct the steering of the clip delivery system towards the TV, with X-plane or live 3D multiplanar reconstruction imaging to optimise the trajectory (Figure 3B).
The importance of transgastric views in the guidance of tricuspid TEER cannot be overemphasised. The transgastric short-axis view (~30°) is used to aid positioning of the clip at the target zone (Figure 3C). This view helps to visualise all the TV leaflets, localise leaflet coaptation gap and regurgitation jet origin. Most importantly, this view enables continuous monitoring of the clip arm orientation to maintain perpendicularity to the line of coaptation. Additionally, the optimal position of the clip and perpendicularity can be assessed by the 3D en face view from a right atrial perspective (Figure 3D).
The midoesophageal ‘commissural’ view with X-plane imaging is used to visualise both arms of the clip during grasping of the leaflets. In the case when the 2D planes are not ‘in-plane’ with the device and leaflets, despite careful probe manipulation, live 3D multiplanar reconstruction enables real-time alignment of the reconstructed orthogonal planes to match device orientation, to monitor leaflet grasping (Figure 3B).
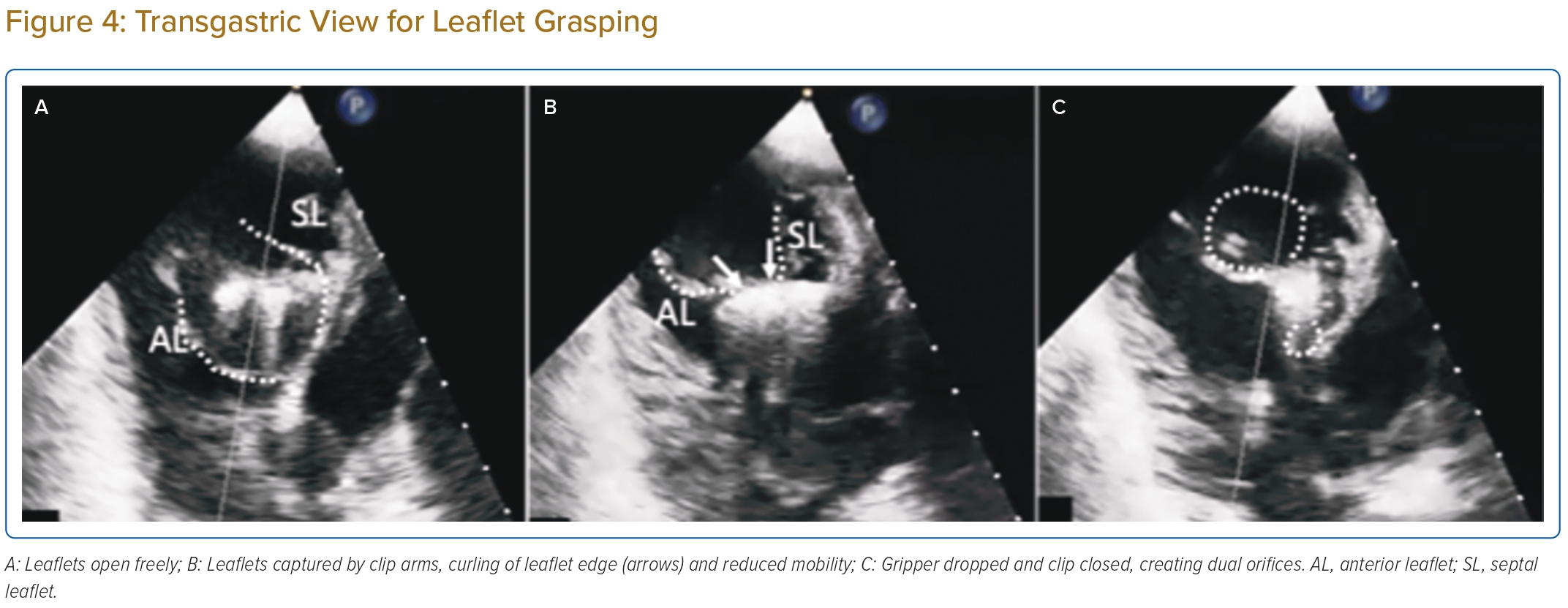
The transgastric short-axis view is useful for leaflet grasping, by observing the in-curling of leaflets and reduction in leaflet mobility, which indicates the leaflets are on clip arms; when this sign is observed, gripper dropping and clip closure will further reduce leaflet mobility creating dual orifices (Figure 4).
Finally, leaflet insertion is assessed by using all available views, including formation of the tissue bridge with clip stability, the amount of leaflet insertion with reference to baseline leaflet length, reduced leaflet mobility and TR improvement. If TOE image quality is insufficient, transthoracic echocardiogram can be used as adjunct to TOE to guide the assessment. When TOE is contraindicated, intracardiac echocardiogram has been used as a complimentary imaging tool.29,30
Conclusion
Although TEER for TR appears to be safe and effective in TR reduction, there are still a lot of uncertainties in the field. First, there are no randomised controlled data on the symptomatic and outcome benefits of TEER compared with optimal medical therapy. The TRILUMINATE and CLASP II TR will try to address this question. Second, there is uncertainty about the appropriate timing of TR intervention relating to a patient’s symptoms, severity of TR, right ventricular function and pulmonary arterial systolic pressure. It is also important to identify clinical and imaging markers of futile TR interventions. Finally, safety and efficacy comparisons of different TEER devices and tricuspid intervention devices will also be needed. 
Clinical Perspective
- Transcatheter edge-to-edge repair of the tricuspid valve is currently the most widely adopted interventional therapy for patients with severe symptomatic tricuspid regurgitation.
- Understanding the severity and mechanisms of tricuspid regurgitation, the anatomy of the tricuspid valve through detailed transthoracic and transoesophageal echocardiogram is important to guide patient selection for transcatheter edge-to-edge repair.
- Successful transcatheter edge-to-edge repair for tricuspid regurgitation relies on optimal communication and collaboration between the implanter and interventional imager.
- A standardised procedural and imaging protocol should be adopted to optimise the interventional result.