Hypertension is one of the main risk factors for cardiovascular diseases, stroke and kidney failure. Approximately 1.28 billion adults worldwide are afflicted by hypertension, predominantly in lower-middle-income countries.1 Among individuals with hypertension, a small subset experience resistant hypertension (RH), a condition that often complicates or impedes hypertension treatment and is associated with a heightened long-term risk of morbidity and mortality (stroke, kidney failure, coronary heart disease and an increased risk of death in patients with congenital heart disease).2,3
RH is diagnosed when a patient has used three different antihypertensive drugs including at least one diuretic but has not achieved the target BP of 140/90 mmHg, or when BP control is achieved but requires ≥4 medications.2 The European Society of Hypertension and the European Society of Cardiology recommend adding one of three drugs – a mineralocorticoid receptor antagonist (MRA), amiloride or doxazosin – in addition to the use of angiotensin-converting enzyme inhibitors (ACEi) or angiotensin receptor blockers (ARBs), calcium channel blockers (CCBs) and thiazide diuretics to manage RH patients.4 The American Heart Association suggests the addition of MRAs, ß-blockers, central α-2 agonists, or direct vasodilators as the fourth antihypertensive agent based on patient characteristics.2 The Eighth Joint National Committee recommends a combination of diuretics, ACEi/ARBs and CCBs, with the addition of a fourth agent, such as a ß-blocker or MRA, to achieve the targeted BP in RH patients.5
These guidelines consider MRAs a key component of RH management. MRAs act by binding to the mineralocorticoid receptor, thereby inhibiting aldosterone and leading to a reduction in BP. Spironolactone and eplerenone, two types of MRAs, are frequently used for hypertension management, including RH, each harbouring distinct advantages and disadvantages. Although both agents have demonstrated effectiveness in reducing BP among hypertensive patients, existing studies predominantly exhibit a favourable trend towards spironolactone for RH management, because of its superior ability to lower BP.6–8 However, it is important to note that these findings primarily stem from non-randomised controlled trials (RCTs) and placebo trials. To the best of the author’s knowledge, no RCTs have directly compared these two drugs and their effectiveness in lowering BP among RH patients.
Given this background, this article describes a systematic literature review and an indirect meta-analysis comparing spironolactone and eplerenone in the context of managing RH patients.
Methods
Literature Search Strategy
The literature search included RCTs comparing eplerenone or spironolactone with placebo in RH patients from 2011 to September 2021 The search strategy used was based on the medical subject heading (MeSH) terms and Boole’s logical operators (Supplementary Data). Searches were limited to the English language and full text. Studies evaluating renal denervation were excluded. A systematic review was performed in the following databases: PubMed, Cochrane, ClinicalTrials.gov, EBSCOHOST, Europe PMC and Google Scholar.
Selection Criteria
Two reviewers (authors HL and WNH) used the same search strategy to identify relevant clinical trials and disagreements were solved with a discussion and by asking the opinion of a third reviewer (D) if necessary. The screening was conducted in two phases: title and abstract review and a full-text review. Each study’s eligibility was based on the following criteria: the studies needed to be RCTs comparing the effects of eplerenone or spironolactone with a placebo in adult patients with RH. RH is defined as uncontrolled BP ≥140/90 mmHg while on optimal doses of at least three antihypertensives, with one being a diuretic or when BP control is achieved but requires ≥4 medications. The selected studies were required to report systolic or diastolic BP measurements before and after the interventions. Abstracts, letters, comments, case reports and editorials were excluded due to their limited provision of detailed information.
Data Extraction and Quality Assessment
Data extraction was conducted in accordance with the PRISMA criteria. Two reviewers (HL and WNH) independently extracted the following information from the studies: year of publication; study location; study design; duration of follow-up; characteristics of the study population (number of patients, mean age, sex, comorbidities, baseline antihypertensives used); details of the interventions (dosage, duration); BP measurements (systolic and diastolic) before and after the intervention; as well as serum potassium levels. Any duplications in studies were identified and grouped to ensure that participants in individual studies were only included once.
The primary outcome was the mean difference in both systolic and diastolic BP between eplerenone and spironolactone. The secondary outcome was the mean difference in serum potassium levels. We independently assessed the quality of the studies using the risk-of-bias assessment tool recommended by the Cochrane Collaboration.9
Statistical Analysis
A pairwise meta-analysis was initially conducted using mean BP and mean potassium level differences as continuous variables. Weighted averages were reported as mean differences +SD with associated CIs using a DerSimonian and Laird random-effects model. Meta-analysis statistics were conducted using Review Manager (RevMan) version 5.6, and MedCalc version 17.6. Statistical significance was determined using a threshold p-value of 0.05 and a 95% CI. The pooled estimates from the meta-analysis were subsequently analysed with a Bucher indirect comparison method using an Excel-based tool developed by Tobias et al.10,11 to attain the adjusted indirect comparisons. Certain population characteristics, including comorbidities, such as type 2 diabetes (T2D), chronic kidney disease (CKD) and heart failure (HF), could potentially influence the outcomes of mean BP and potassium differences. To evaluate whether these variables affected the outcomes, subgroup analyses were conducted using RevMan version 5.6.
Statistical heterogeneity for each pairwise comparison was assessed using the I2 statistic. I2 values were calculated to assess the variability in intervention effects across studies. Based on I2, heterogeneity was categorised as low (<25%), moderate (25–75%), or high (>75%).
Results
Search Results
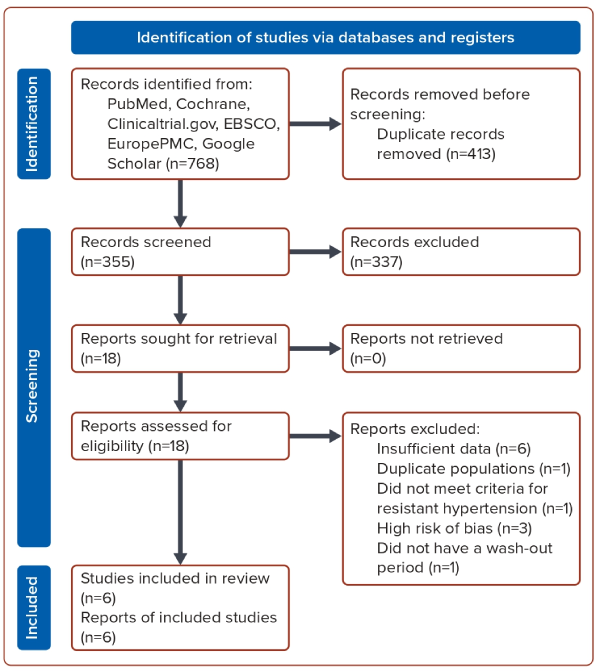
Initial search terms resulted in 768 studies which underwent further screening. Following the initial screening, 337 studies were excluded for the following reasons: they were not RCTs; they were single-arm trials; they did not use a placebo as a comparator; they were not written in English; or they were not conducted in humans. Further screening resulted in 18 unique studies that were retrieved and underwent full-text assessment. Among these, eight were excluded due to factors, such as duplicate populations, insufficiently detailed BP data or failure to meet our criteria for RH. This left 10 studies, two of which were subsequently excluded — one due to an incomplete dataset and the other for not having a wash-out period between drugs (Figure 1).12,13
The remaining eight studies were evaluated to have a high-to-low risk of bias (using the Cochrane Risk of Bias tool) based on these factors: having appropriate randomisation; being double-blinded studies; having adequate concealment of treatment allocation; having care providers, participants and outcome assessors blinded to treatment allocation; having similar prognostic groups at the start of the study; using intention to treat analysis; and having appropriate methods to account for missing data. Two studies fell into the high-risk criteria. Among these, two studies either lacked appropriate randomisation or exhibited inadequate concealment of treatment allocation (Supplementary Figure 1). Consequently, six studies met the criteria for inclusion in the final review.
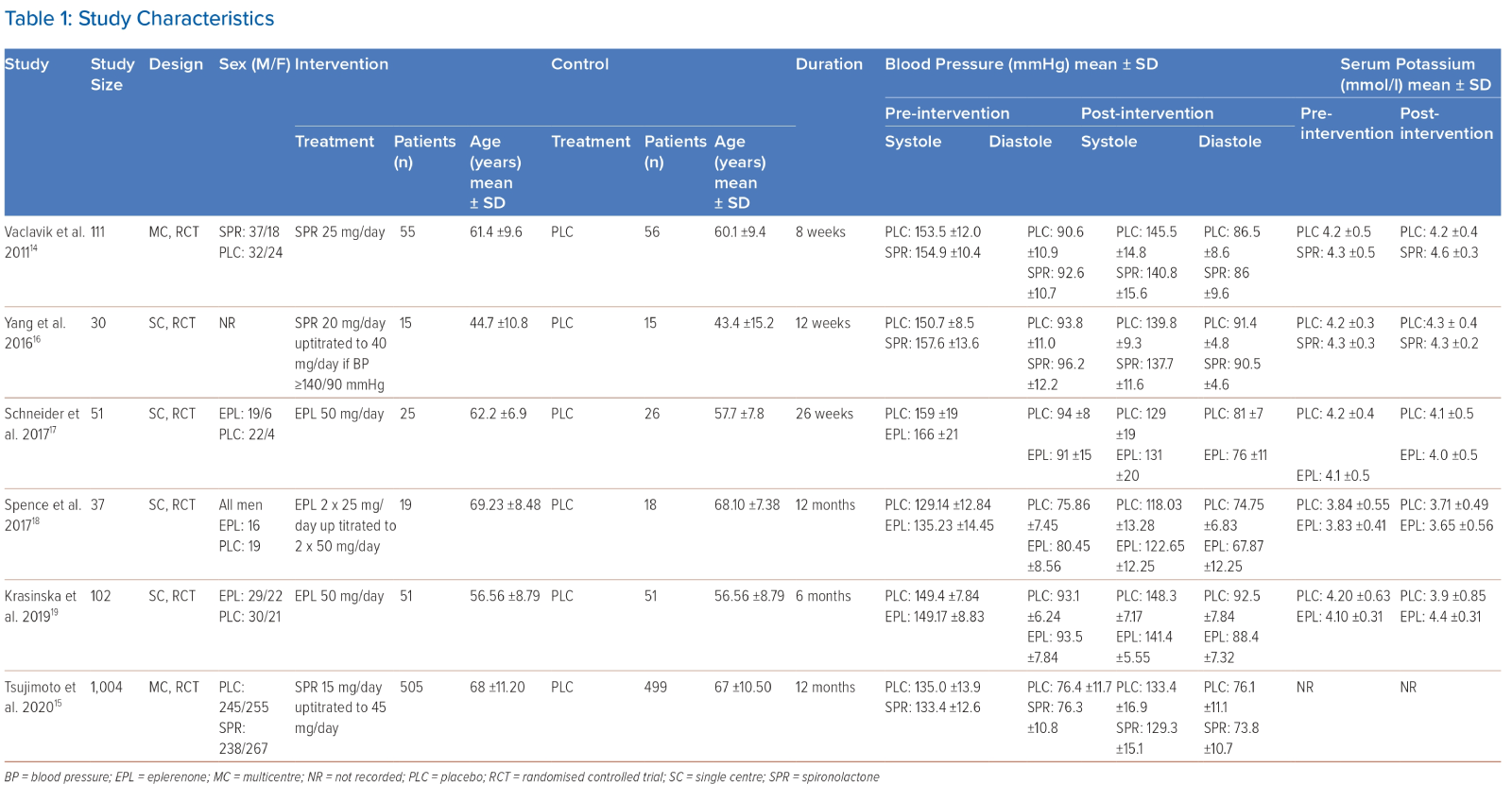
A description of each study can be seen in Table 1. Six RCT studies were included in the systematic review, which was evenly divided between studies involving spironolactone and those involving eplerenone. Among the six studies, two were conducted across multiple centres, while the remaining four were single-centre studies. Of these, three studies had sample sizes exceeding 100 patients, whereas the remaining three studies involved fewer than 100 patients.
The initial dose of the drug used in the spironolactone group appears varied. The study by Vaclavik et al. used a starting dose of 25 mg/day of spironolactone, the study by Yang et al. initiated treatment at 20 mg/day and Tsjumito et al. at 15 mg/day.14–16 All studies in the eplerenone group used a 50 mg/day starting dose.17–19 The duration of the study for each trial ranged from 8 weeks to 12 months.
The mean number of baseline antihypertensives used in both the spironolactone and eplerenone groups is four, primarily comprising a combination of ACEIs/ARBs, CCBs, ß-blockers, α-blockers, centrally acting hypertensives, and/or diuretics. All medications were continued throughout the follow-up period. Although the doses of each drug were not reported in the included studies, there were no significant differences in the distribution of the basic antihypertensives used.
Among the studies in the spironolactone group, Yang et al. demonstrated the most substantial reduction in systolic and diastolic BP following the spironolactone intervention, measuring at −19.9 ± 13.4 mmHg and −5.7 ± 12.5 mmHg, respectively.16 In contrast, within the eplerenone group, Schneider et al. exhibited the highest post-interventional reduction in systolic and diastolic BP, registering at −35 ± 20 mmHg and −15 ± 11 mmHg, respectively.17 Conversely, there were no significant disparities observed in serum potassium levels in both groups.
Two out of three studies reported that all patients achieved well-controlled BP with the addition of eplerenone.17,18 However, the study conducted by Krasinska et al. employed a distinct study design, deliberately refraining from titrating the eplerenone dose to attain target BP.19 Conversely, within the spironolactone group, only one study reported the percentage of patients achieving well-controlled BP. In this study, 30 patients (54.5%) using spironolactone attained target systolic BP (p=0.25) and 38 patients (69.1%) achieved target diastolic BP (p=0.68).14
According to available literature, adverse events were observed in some patients within the MRA group. Spence et al. demonstrated that 18.2% of patients experienced potassium levels ≥5.5 mEq/l (p=0.53), with one patient experiencing levels >6 mEq/l (p=0.74).18 Furthermore, 77.3% of patients on eplerenone reported minor adverse events during any visit. In a study conducted by Tsujimoto et al., 7.92% of patients taking spironolactone experienced an elevation in serum potassium levels to ≥5.5 mEq/l (p<0.001) and 3.3% reached levels ≥6 (p<0.001).15 Similar trends were reported by Vaclavik et al., where 43% of patients experienced adverse events, leading to a discontinuation of spironolactone in 3.6% of cases due to severe adverse events.
Indirect Meta-analysis
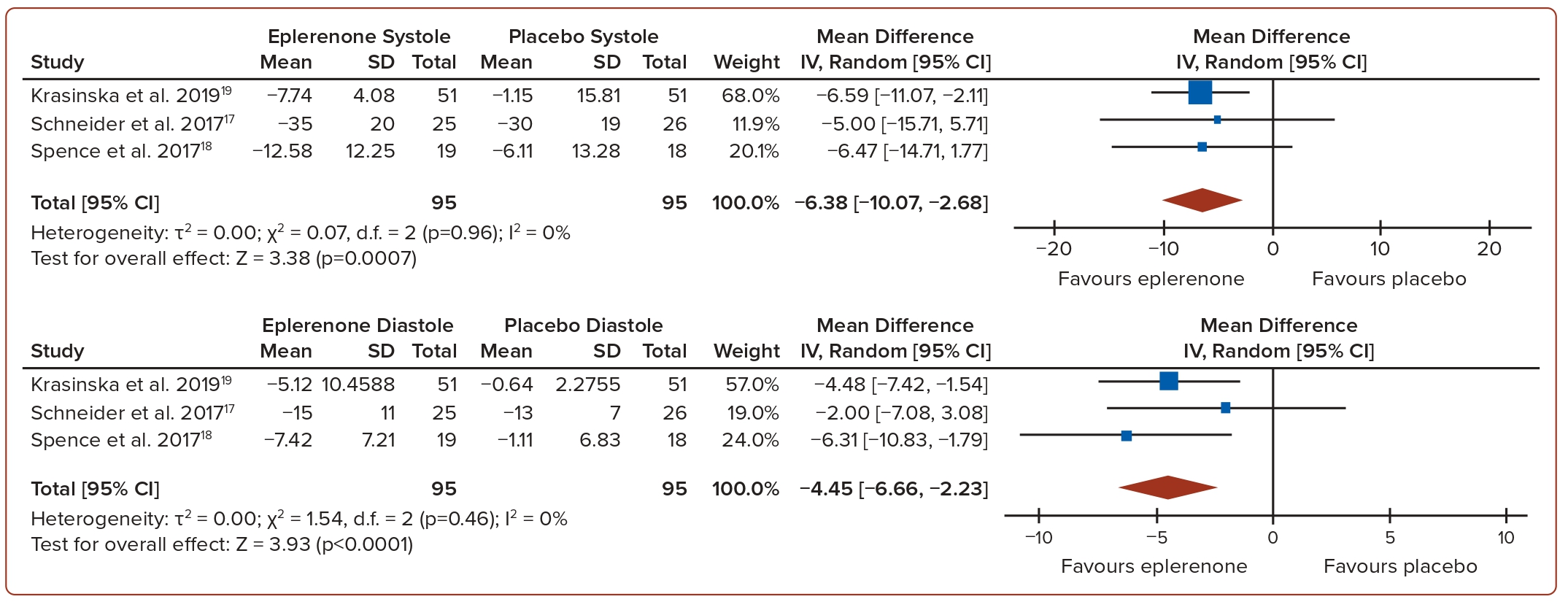
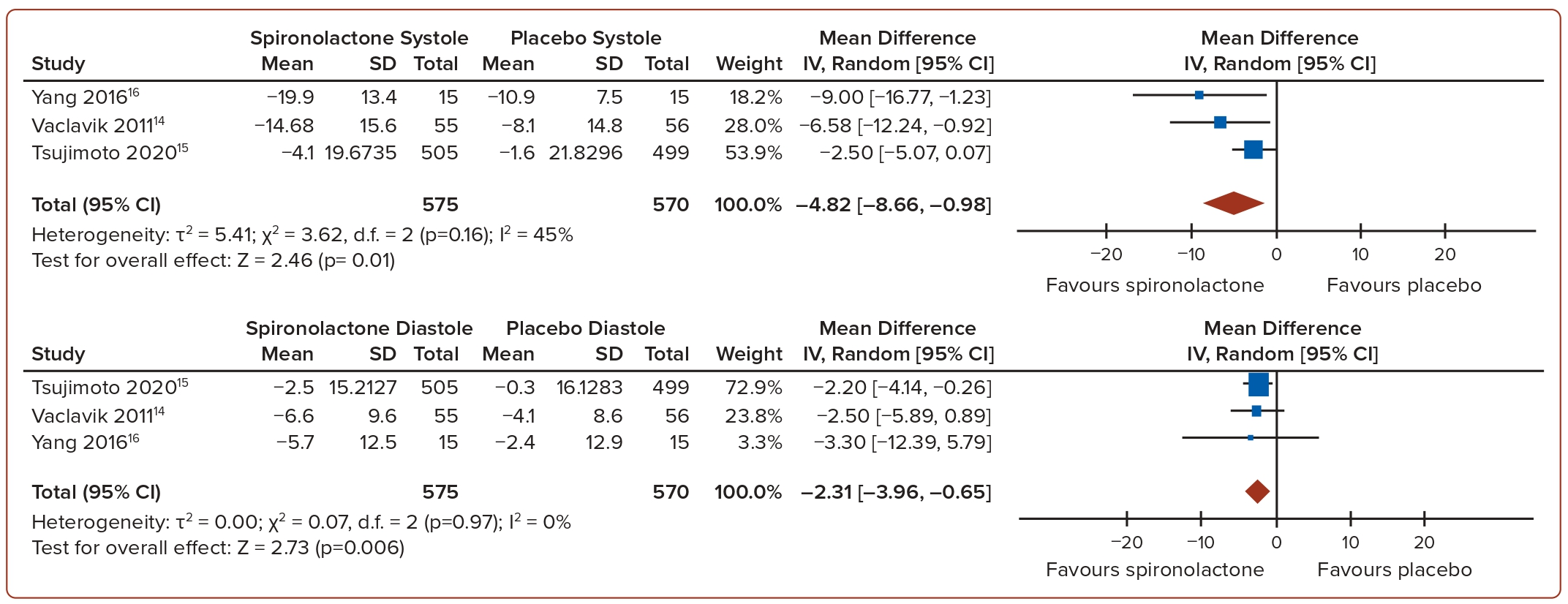
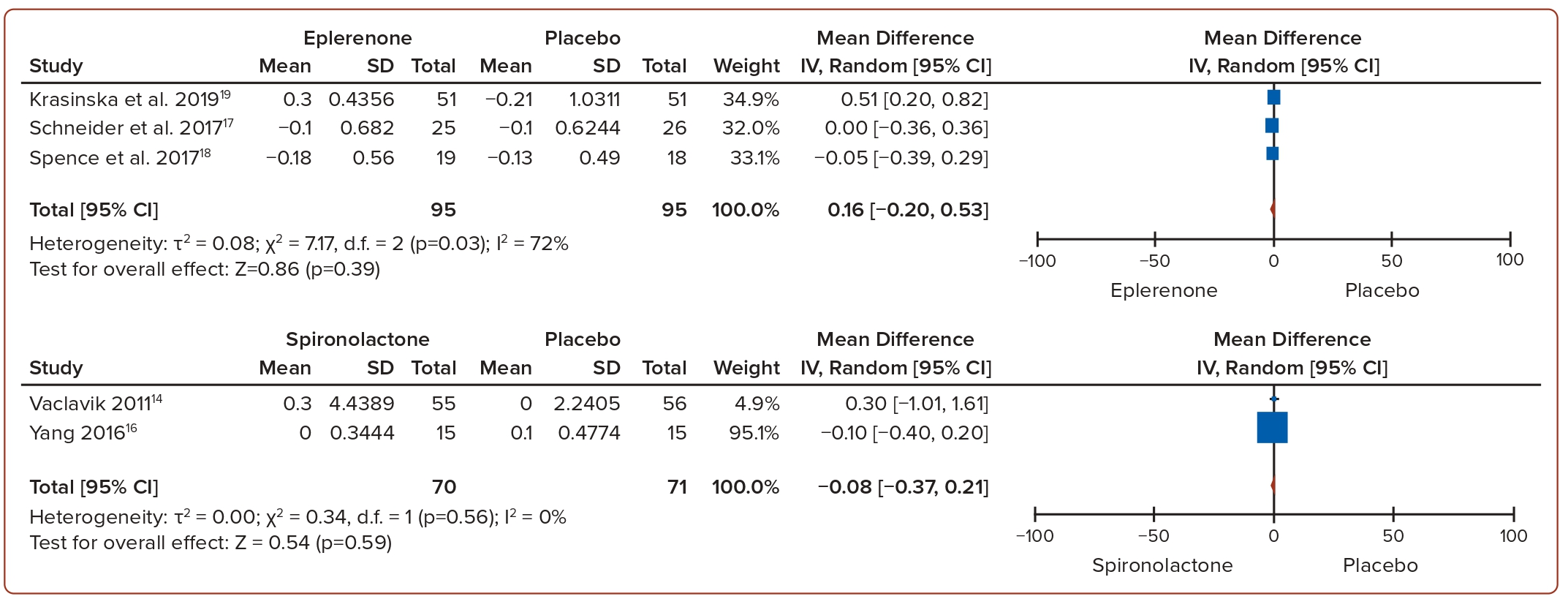
The pooled mean difference of systolic and diastolic BP between eplerenone and placebo showed a decrease in systolic BP of −6.38 mmHg (p=0.0007, I2=0%) and −4.45 mmHg (p<0.0001, I2=0%), respectively (Figure 2). Similarly, the pooled mean difference of systolic and diastolic BP between spironolactone and placebo showed a decrease in systolic BP of −4.82 mmHg (p=0.01, I2=45%) and a decrease in diastolic BP of −2.31 mmHg (p=0.006, I2=0%; Figure 3). In general, the use of MRAs led to a more significant reduction in both systolic and diastolic BP compared to a placebo. In terms of serum potassium levels, the pooled MD between eplerenone and placebo was 0.16 mmol/l (p=0.39, I2=72%), while for spironolactone versus placebo, it was −0.08 mmol/l (p=0.59, I2=0%; Figure 4).
The indirect comparison meta-analyses of the mean difference in systolic BP between spironolactone and eplerenone revealed no statistically significant difference between the two groups (MD 1.66; 95% CI [−3.46, 6.78]; p=0.525). A similar trend can be observed in the indirect comparison of the MD in diastolic BP (MD 2.14; 95% CI [−0.63 to 4.91]; p=0.129; Table 2).
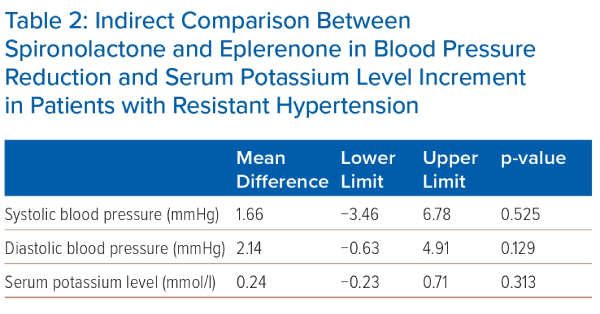
In relation to mean serum potassium levels, the indirect comparison between spironolactone and eplerenone demonstrated no statistically significant difference (MD 0.24; 95% CI [−0.23 to 0.71]; p=0.313; Table 2). While not achieving statistical significance, it is worth mentioning that eplerenone elicited a higher incidence of hyperkalaemia when compared to spironolactone.
Subgroup Analysis
Subgroup analyses were conducted to investigate the heterogeneity present within the included studies, particularly focusing on the spironolactone studies. Key study characteristics considered were T2D, HF and CKD. The analysis within the T2D subgroup did not indicate T2D as a likely cause for the observed high heterogeneity (p=0.23, I2=30.8%) (Supplementary Figure 2). A similar outcome was observed within the CKD subgroup (p=0.85, I2=0%) and the HF subgroup (p=0.07, I2=70.6%) (Supplementary Figures 3 and 4). From a clinical perspective, no definite potential sources of heterogeneity could be ascertained, which may have resulted from the methodological diversity across the studies. Meta-regression analyses were not performed due to the limited number of studies included.
Discussion
This study aimed to evaluate the difference between spironolactone and eplerenone regarding their effectiveness in lowering BP among patients with RH. The analyses of six RCTs yielded results indicating that there is no notable difference between spironolactone and eplerenone in terms of the capacity to lower systolic and diastolic BP in patients with RH. Moreover, the secondary outcome analysis also indicated a lack of significant disparity in serum potassium levels following the administration of these two medications. Notably, these findings represent the first indirect comparison meta-analyses conducted between the two MRA agents, offering novel insights into their relative efficacy.
To the best of our knowledge, there are no RCTs that directly compare spironolactone and eplerenone. In a prospective study conducted in 2018 involving 180 patients with HF, an attempt was made to assess the comparative efficacy of spironolactone and eplerenone. The mean dosage for each MRA was reported as 28 ± 10 mg for spironolactone and 34 ± 15 mg for eplerenone. The outcomes of a 1-year follow-up revealed no significant disparity in changes to systolic BP between the two agents.20 Conversely, a separate previous study investigated the impact of BP reduction after the administration of eplerenone (ranging from 50 to 400 mg/day) versus placebo, using spironolactone 50 mg/day as a control. The study noted that alterations in BP from baseline seemed more pronounced with spironolactone in comparison to eplerenone, indicating a potential superiority of spironolactone. It’s important to emphasise, however, that this study did not undertake a statistical analysis comparing eplerenone and spironolactone.21
Existing evidence suggests that MRAs may offer substantial benefits to patients with RH, particularly in achieving optimal BP levels. Interestingly, the observed reduction in BP appears to be more prominent in individuals with uncontrolled RH compared to those who have already attained the target BP.15,17,18 Paradoxically, the introduction of MRAs to patients with controlled RH has demonstrated a reduced need for additional antihypertensive medications and/or a decrease in their dosages to achieve the desired BP target. This, in turn, leads to a mitigation of the side-effects associated with other antihypertensive agents.17,18 Such findings underscore the potential dual benefits of MRAs in not only enhancing BP control in RH but also in minimising the overall burden of antihypertensive medications and their associated adverse effects.
It appears that the duration of MRA use does not appear to correlate linearly with the reduction of BP in patients with RH which can be seen in the varying endpoint BP reduction between the included studies. Moreover, the majority of these studies lacked multiple follow-up period checkpoints, making it challenging to assess the correlation between treatment duration and BP reduction.13–18 However, one study demonstrated that the peak reduction in systolic and diastolic BP occurs in the fourth month of medication and remains stable for the remainder of the follow-up period. Despite this plateau in BP reduction, the rates of major adverse cardiovascular events, hospitalisation for HF and all-cause death decrease proportionately over time.15
Additionally, Schneider et al. suggested that eplerenone exhibits BP-independent effects that contribute to the reduction of ventricle mass, ultimately associated with a diminished risk of cardiovascular disease despite the modest difference in BP reduction compared to the placebo.17 This emphasises that the cardiovascular benefits of MRA therapy may extend beyond mere BP reduction, particularly in populations with specific cardiovascular risk profiles.
Hyperkalaemia is an important monitoring parameter for patients with RH undergoing treatment with MRA agents. Although previous studies have hinted that spironolactone may be associated with a higher incidence of hyperkalaemia and its complications due to its longer half-life, our present study does not reveal a significant difference in serum potassium levels between the two agents.21,22
These findings align with the observations made by Yamamoto et al., where the mean difference in serum potassium levels between the two agents exhibited minimal variation (spironolactone = 0.0 ± 0.6 versus eplerenone 0.1 ± 0.5; p=0.510).20 However, it is crucial to note that the aforementioned study encompassed patients without RH, thereby necessitating careful interpretation of its implications in the context of our investigation.
The use of MRAs, specifically spironolactone as opposed to eplerenone, is associated with additional relevant side-effects. In an eplerenone study, various minor adverse events were reported in 77.3% of patients, although no discernible pattern suggested a causal relationship to eplerenone. However, only one patient (4.7%) discontinued the medication due to severe hyperkalaemia.18
Conversely, two studies involving spironolactone revealed side-effects directly attributable to this agent. These included gynaecomastia and/or mastodynia in 3.3% of patients (n=9) and an anaphylactoid reaction in 0.19% of patients (n=1).15 Notably, one study proposed vertigo as a potential side-effect of spironolactone, occurring in 14.5% of patients, although it was comparable to the incidence observed in the placebo group.14 These findings underscore the importance of distinguishing between specific MRAs when considering potential side-effects, with spironolactone demonstrating a distinct profile in comparison to eplerenone. Although most side-effects were minor and did not necessitate discontinuation, careful evaluation and monitoring are warranted to assess and manage these side-effects appropriately in clinical practice.
Given the relatively comparable BP-lowering effects of the two drugs, the decision regarding which MRA to choose to treat patients with RH hinges on other factors. Pharmacologically, both medications work by inhibiting aldosterone by binding to the mineralocorticoid receptor. However, spironolactone has a higher affinity for progesterone and androgen receptors, leading to side-effects, such as gynaecomastia, sexual dysfunction and mastodynia.21 Additionally, eplerenone does not interfere with the body’s metabolic processes, making it a preferable option for patients with metabolic disorders.21–23 However, the potential drawback lies in its higher cost. Achieving the optimal dosage for hypertension treatment could translate into eplerenone costing six- to seven-times more than spironolactone within 1 month.24 Nevertheless, it is noteworthy that while certain studies have demonstrated the cost-effectiveness of eplerenone in patients with HF, a corresponding body of research is currently lacking for patients with RH.25
Study Limitations
Our study is subject to certain limitations, primarily stemming from the heterogeneity among the included trials especially in the spironolactone group. Subgroup analysis cannot precisely explain the source of the heterogeneity, which may have resulted from differences in population characteristics and study designs. Consequently, the generalisability of the results to real-world clinical practice is limited, necessitating caution in the interpretation of findings. Therefore, while our study provides valuable insights, its application to broader clinical contexts should be approached with careful consideration of the inherent heterogeneity.
Moreover, a limitation arises from a lack of studies incorporating 24-hour ambulatory BP monitoring or home BP monitoring, which could provide a more precise and robust BP assessment. Nonetheless, reliance on office BP readings still offers a reasonable depiction of the effects of the two agents on BP in RH patients.
Future research endeavours with more homogenous participant characteristics and study designs may contribute to a more robust and widely applicable evidence base and has the potential to enhance the validity and generalisability of our findings.
Conclusion
In this review, both spironolactone and eplerenone demonstrated an equivalent reduction in systolic and diastolic BP and serum potassium concentration among patients with RH. Further investigations, ideally through direct head-to-head comparisons, are imperative to provide a more comprehensive understanding and validation of these findings. 
Click here to view Supplementary Material
Clinical Perspective
- Spironolactone and eplerenone demonstrated an equal reduction in systolic and diastolic blood pressure and serum potassium levels in patients with resistant hypertension.
- These findings may assist clinicians in selecting a personalised mineralocorticoid receptor antagonist for patients with resistant hypertension, with a greater emphasis on other factors, such as side-effects, metabolic profile and cost-effectiveness.