Introduction
Combination treatment with veno-arterial extracorporeal membrane oxygenation (VA-ECMO) and Impella (ECPELLA) has been recently proposed for patients with cardiogenic shock (CS) exacerbated by biventricular cardiac dysfunction. This could simultaneously achieve a significant reduction in preload for both ventricles by right atrial (RA) venting with VA-ECMO and left ventricular venting with Impella, and could improve organ perfusion by improving blood flow through the aorta.1 ECPELLA has been reported to reduce the use and dose of intravenous inotropes in patients with CS, as well as reducing 30-day mortality by 20–25% compared with VA-ECMO alone.2,3 However, even in patients treated with ECPELLA, the 30-day mortality rate remained above 50%, which could be further improved.2,3
Patients requiring ECPELLA include those with extracorporeal cardiopulmonary resuscitation, those with hypoxaemia and those with severe right ventricular dysfunction with VA-ECMO indications, in addition to left ventricular dysfunction with Impella indications.4 Treatment of the underlying disease, including early reperfusion therapy for acute MI for myocardial recovery, should be appropriately performed. Nevertheless, in cases of broad myocardial ischaemia, myocarditis and worsening chronic heart failure, right ventricular dysfunction could remain, and the weaning of patients off VA-ECMO may be prolonged.5,6 In the present study we focused on the treatment strategies for ECPELLA patients with prolonged right ventricular dysfunction. Treatment options for patients with right ventricular dysfunction include the addition of an extracorporeal right ventricular assist device, Impella heart pump (Impella RP) and pharmacotherapy.7 Pharmacotherapy for patients with right ventricular dysfunction includes fluid loading, intravenous inotropes and pulmonary vasodilators.
Inhaled nitric oxide (iNO) is a pulmonary vasodilator that can be incorporated into the ventilator and can be administered by inhalation. iNO is taken up transalveolarly into the pulmonary capillaries, reducing pulmonary vascular resistance, increasing pulmonary blood flow and reducing the right ventricular afterload.8 We focused on the haemodynamic effects of iNO in CS patients during ECPELLA support. We hypothesised that the introduction of iNO concurrently with ECPELLA support in patients with prolonged right ventricular dysfunction would improve transpulmonary blood flow and right ventricular performance, which could improve the weaning process of patients off VA-ECMO. The purpose of the present study was to review and characterise the haemodynamic effects of iNO introduction in CS patients treated with ECPELLA.
Methods
Study Patients
The present study was a single-centre retrospective observational study conducted on Japanese patients with CS. Consecutive patients who were started on Impella with a diagnosis of CS and were treated with iNO during Impella support between January 2019 and May 2021 were enrolled. Patients who did not undergo a haemodynamic evaluation by pulmonary artery (PA) catheterisation and those who underwent iNO introduction at the same time as starting Impella support were considered unsuitable for the evaluation of the effects of iNO and were excluded. The purpose of iNO introduction and the doses used were left to the discretion of the attending physicians. Because iNO has been approved in Japan for improving pulmonary hypertension perioperatively during cardiac surgery, it was introduced when the physician considered the clinical condition to be equivalent in the present study. The clinical condition was comprehensively assessed by PA pressure, pulmonary vascular resistance (PVR) and right heart function. Impella P levels were adjusted at the discretion of each physician and did not have an algorithm that allows them to be adjusted automatically.
The present study was approved by the Kitasato University Hospital Institutional Review Board, and an opt-out method was used to obtain consent from patients.
Study Protocol
Patient demographics, the aetiology of CS, concomitant treatments, including medications and the auxiliary flow rate of mechanical circulatory support (MCS), outcomes during weaning off Impella, echocardiographic parameters, heart rate, arterial pressure, PA catheter data and the ratio of PaO2/fraction of inspired oxygen (P/F ratio) were obtained immediately before iNO introduction (baseline [BL]). In addition, haemodynamic parameters, auxiliary flow rate of MCS and the P/F ratio were obtained 24 hours before (–24 hours), 12 hours before (–12 hours), 12 hours after (+12 hours) and 24 hours (+24 hours) after iNO introduction. Patients were divided into two groups: those receiving ECPELLA and those receiving Impella alone.
Measurements
Medical records were reviewed to obtain patient characteristics. All PA catheter data, including pressure data and cardiac output (CO) by the thermodilution method, were obtained at rest during ongoing treatment without drug testing or exercise stress testing. The PA pulsatility index and right ventricular stroke work index (RVSWI) were calculated according to the following formulas:9,10
PA pulsatility index = (PA systolic pressure − PA diastolic pressure)/RA pressure
RVSWI = (PA mean pressure − RA pressure) × stroke volume index
where RA is right atrium. The presence of right ventricular dysfunction was determined as reported previous.10 Echocardiography was performed transthoracically; M-mode images in left parasternal long-axis views were obtained to measure the dimension of each chamber and biplane 2D images from apical viewpoints were obtained to calculate the left ventricular ejection fraction based on the modified Simpson’s method.11
Statistical Analysis
Continuous variables are presented as the mean ± SD or median with range (minimum–maximum) and categorical variables are presented as frequencies and percentages, as appropriate. For comparisons between groups, the Wilcoxon rank-sum test was used for continuous variables and Fisher’s exact test was used for categorical variables. For comparisons of variables between different time points in the same individual, a paired t-test was used. Statistical significance was set at p<0.05. All statistical analyses were performed using JMP 13 (SAS Institute).
Results
Patient Characteristics
Eighty consecutive patients who received Impella support and had a diagnosis of CS were screened for inclusion in this study. Eleven patients with iNO therapy during Impella support were enrolled in the study after excluding 67 patients without iNO therapy, one patient for whom PA catheter data were unavailable and one patient in whom iNO was introduced simultaneously with Impella support. Of the 11 patients included in the study, eight were treated with ECPELLA and three patients were treated with Impella alone. All ECPELLA patients had VA-ECMO and Impella initiated simultaneously. The patients’ characteristics are presented in Table 1. The causes of CS were MI in six patients, heart failure deterioration in three patients and myocarditis in two patients. iNO was started in patients a median of 5 days (range 1–10 days) after the introduction of Impella support at a median maximum dose of 20 ppm (range 20–40 ppm). Most patients were treated with concurrent intravenous inotropes and diuretics at BL.
Baseline Clinical Parameters
The baseline echocardiographic and haemodynamic indices are presented in Supplementary Tables 1 and 2. Patients with ECPELLA had shorter left ventricular end-diastolic and end-systolic diameters and lower tricuspid annular plane systolic excursions, and patients with ECPELLA also had a higher RA pressure, lower PA pulsatility index and lower RVSWI. All patients with ECPELLA and two patients with Impella alone had right ventricular dysfunction at BL.
Clinical Parameters Before and After Introduction of Inhaled Nitric Oxide
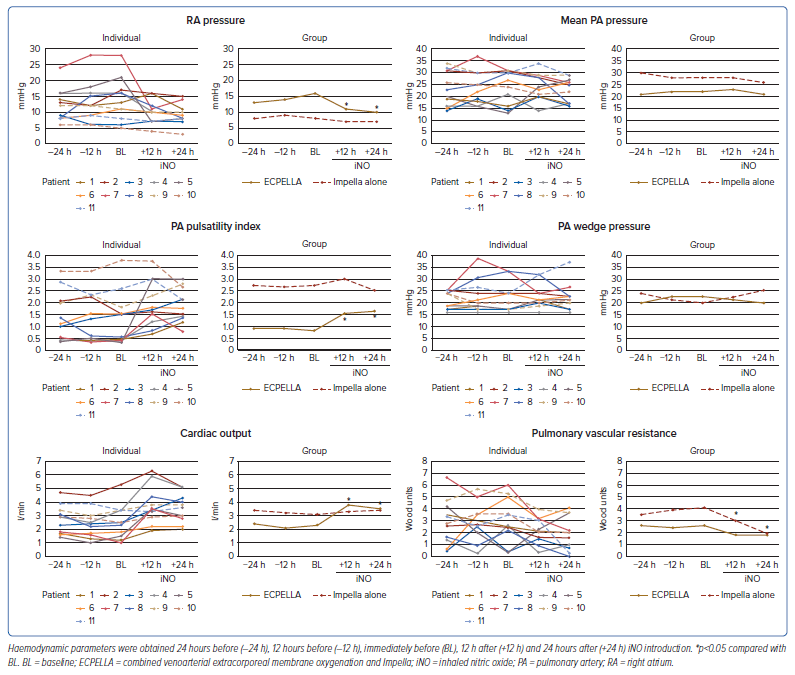
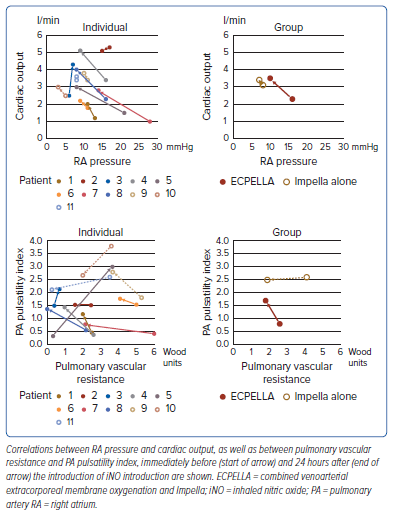
After iNO introduction, the aortic pulse pressure and pulmonary pulse pressure were increased in patients with ECPELLA, as shown in Supplementary Figure 1. Changes in PA catheter parameters are shown in Figure 1. In patients with ECPELLA, RA pressure decreased and the PA pulsatility index and CO increased, whereas no significant changes were demonstrated in these parameters in patients with Impella alone. The mean PA pressure and PA wedge pressure were not significantly different in either group. Only Patient 11 showed a marked increase in the PA wedge pressure and a deterioration of pulmonary congestion on chest X-ray after iNO introduction (Supplementary Figure 2). The PVR was markedly decreased in patients with Impella alone. Correlations between RA pressure and CO, as well as between PVR and the PA pulsatility index, before and after iNO introduction in individual patients and in the ECPELLA and Impella groups as a whole are summarised in Figure 2.
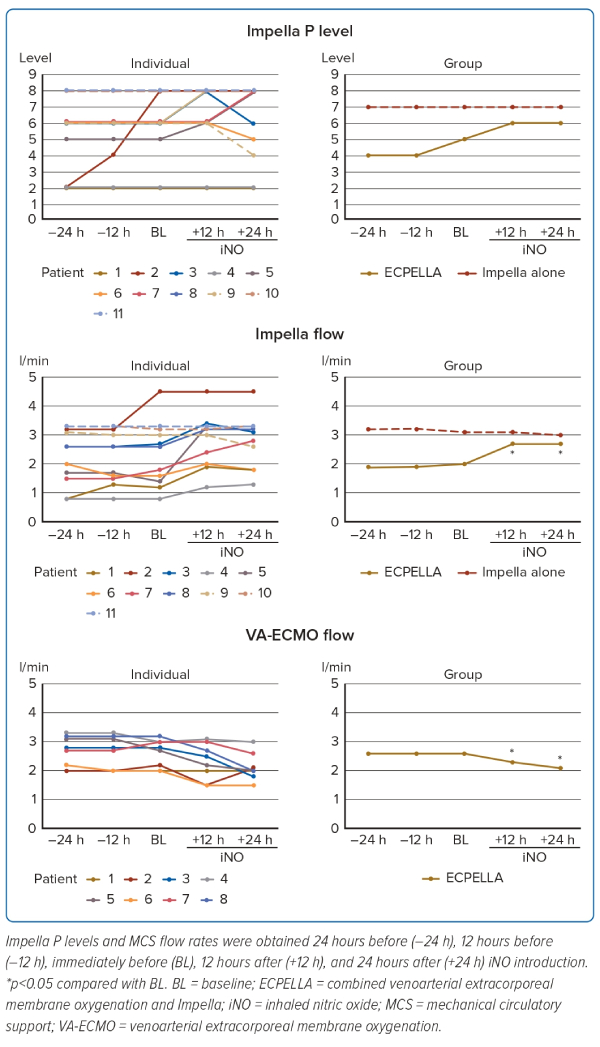
Changes in the MCS auxiliary flow rate are shown in Figure 3. In patients with ECPELLA, the Impella flow rate increased and the VA-ECMO decreased. Three patients with ECPELLA had elevated maximum P levels without causing suction. Changes in the P/F ratio are shown in Supplementary Figure 3. The P/F ratio did not change significantly from before to after the introduction of iNO; only Patient 11 showed a marked decrease in the P/F ratio at +24 hours.
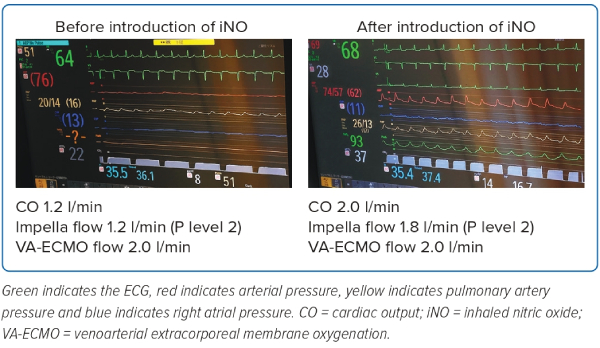
The monitoring screens in Patient 1, the representative case in the present study, at BL and +24 hours are shown in Figure 4. There was an improvement in the poor PA pulsatility and the elevated RA pressure accompanied by an increment in aortic pulse pressure and CO after the introduction of iNO. Moreover, the Impella auxiliary flow rate increased after iNO introduction (the Impella flow rate ranged from 1.2 to 1.8 l/min at P level 2), and the maximum P level without causing suction increased from P level 2 to P level 6 (the Impella flow rate was 1.2 l/min at P level 2, increasing to 2.5 l/min at P level 6).
Pulmonary Vasodilators
Some patients switched from iNO to the temporary use of oral pulmonary vasodilators to avoid the rebound phenomenon during iNO withdrawal. iNO was administered for a median of 6 days (range 2–12 days). Six patients were switched to sildenafil and two patients were switched to tadalafil. Patient 11 discontinued iNO due to exacerbation of pulmonary congestion after iNO introduction, but rapidly improved with renal replacement therapy and diuretics. There was no occurrence of methaemoglobinaemia with the introduction of iNO in any of the patients.
Discussion
Major Findings
The key findings of the present study are that the introduction of iNO in patients with biventricular impairment supported by ECPELLA resulted in an increase in the PA pulsatility index and CO, no significant exacerbation of pulmonary congestion and an increase in the Impella flow rate without causing suction. Although iNO has been already a widely used in clinical settings, the present study is the first to reveal changes in haemodynamic indices after introduction of iNO in patients with ECPELLA. The present study proposed a useful therapeutic strategy using additive iNO for CS patients supported by ECPELLA. The introduction of iNO concurrently with ECPELLA support in patients with right ventricular dysfunction could improve right ventricular performance, which could facilitate weaning off VA-ECMO. In contrast, the marked reduction in PVR was the only characteristic haemodynamic change in patients with Impella alone.
Favourable Effects of Inhaled Nitric Oxide
iNO has been widely used in the management of pulmonary hypertension and right heart failure after heart transplantation, heart valve surgery and the implantation of a left ventricular assist device (LVAD).12–20 The introduction of iNO in postoperative LVAD patients has been shown to improve transpulmonary blood flow, which can improve right ventricular performance.15–19 These haemodynamic effects are not limited to patients with pulmonary hypertension or an elevated PVR.16,21 Although inotropes increase myocardial oxygen demand, iNO is characterised by its ability as a pulmonary vasodilator to increase right ventricular performance without increasing oxygen demand.22 In addition, the increase in the PA wedge pressure can be suppressed by increasing the LVAD flow rate.21 Similar haemodynamic improvements in right ventricular performance seen after the introduction of iNO in postoperative LVAD patients were observed in the present study after iNO was introduced in patients with ECPELLA.
The reduction in right ventricular afterload after introduction of iNO may improve right ventricular performance and could contribute to the weaning process of VA-ECMO. Impella could suppress the excessive increase in PA wedge pressure and could allow the transition to Impella-dependent haemodynamics. The present study proposed that iNO would be expected to be effective in patients with prolonged right ventricular dysfunction with reduced transpulmonary blood flow and an insufficient Impella flow. Improvement of the PA pulsatility index, CO and Impella flow rate could be expected in patients with worse indices, such as in Patient 1. The aortic pulse pressure also increased after iNO introduction, and this was probably due to an increase in left ventricular preload because of the improved transpulmonary blood flow. In contrast, although the clinical benefit of iNO was limited to patients with Impella alone, the PVR was reduced after the introduction of iNO. In cases of pulmonary hypertension and/or right heart failure accompanied by elevated PVR and an inability to increase the Impella flow rate sufficiently, iNO may be effective. Because all patients receiving Impella support alone in the present study were being supported with the Impella CP, the results may be different in patients supported with the Impella 5.0/5.5. The present study summarised the correlations between RA pressure and CO, as well as between PVR and the PA pulsatility index, before and after the introduction of iNO, demonstrating differences in the haemodynamic response between ECPELLA patients and those with Impella alone. In patients with ECPELLA who have impaired right ventricular function, the introduction of iNO improved right ventricular performance independent of the elevation in PVR at BL.
Adverse Effects of Inhaled Nitric Oxide
The problem with pulmonary vasodilation in patients with underlying left heart disease is the concern regarding exacerbation of pulmonary congestion. The introduction of iNO for pulmonary hypertension after cardiac surgery has been associated with a lower risk of reduction in the P/F ratio than that for sildenafil, which is an oral pulmonary vasodilator.23 iNO selectively acts on pulmonary capillaries in ventilated alveoli and avoids the exacerbation of ventilation–perfusion mismatch.24 Nevertheless, in patients with severe left ventricular dysfunction, the risk of worsening the pulmonary congestion due to increased pulmonary blood flow associated with the introduction of iNO should be considered.
In the present study, none of the patients with ECPELLA experienced exacerbation of pulmonary congestion, and there was no notable worsening of the PA wedge pressure or the P/F ratio. The risk of pulmonary congestion was considered to be low in patients with ECPELLA because iNO was introduced with a reduced pulmonary blood flow due to VA-ECMO and concurrent right ventricular dysfunction. In contrast, one of the patients with Impella alone (Patient 11) experienced exacerbation of pulmonary congestion, an increased PA wedge pressure and a decreased P/F ratio after the introduction of iNO. If left ventricular venting is insufficient against increases in the pulmonary blood flow, there could be a risk of worsening pulmonary congestion. The present study found limited effectiveness of the introduction of additive iNO in patients receiving Impella alone. The decision to use iNO should be based on a comprehensive assessment of the fluid volume, left ventricular venting rate of Impella, autologous left ventricular function, PVR and right ventricular function, with particular attention of the possible risk of exacerbation of pulmonary congestion. Finally, the present study highlighted the importance of strict PA catheter monitoring during ECPELLA support, as reported previously.25 Haemodynamic monitoring using a PA catheter should be effective in assessing right ventricular function and PVR to identify patients who are candidates for the introduction of iNO. The findings of the present study also suggest that PA wedge pressure monitoring can provide a preliminary assessment of the risk of pulmonary congestion prior to iNO administration, as well as early detection of pulmonary congestion after the use of iNO.
Limitations
The present study has some considerable limitations. Because the data were taken from a small number of patients at a single centre using a retrospective study design, the possibility of selection bias cannot be excluded. In particular, the very small number of patients with Impella alone severely limits the conclusions that can be drawn in comparison to patients with ECPELLA. The introduction of iNO was not standardised in terms of the patient background, clinical condition or introduction protocol. No control group was established to validate the clinical efficacy of iNO, and changes in clinical parameters from BL were evaluated. In addition, the influence of MCS flow settings, concurrent therapy and spontaneous cardiac recovery should be considered. A prospective controlled study with a uniform protocol is needed in the future.
Conclusion
iNO improved right ventricular function and increased transpulmonary blood flow in CS patients receiving ECPELLA. We demonstrated a useful therapeutic strategy with the use of additive iNO for patients with right ventricular dysfunction supported by ECPELLA.
Click here to view Supplementary Material.
Clinical Perspective
- iNO reduces the right ventricular afterload via pulmonary vasodilation.
- The clinical significance of iNO on right ventricular performance was evaluated in patients with cardiogenic shock treated with ECPELLA.
- The pulmonary artery pulsatility index and Impella flow rate were both ameliorated after iNO introduction.
- The additive introduction of iNO is thought to be a useful therapeutic option for patients with right ventricular dysfunction supported by ECPELLA.