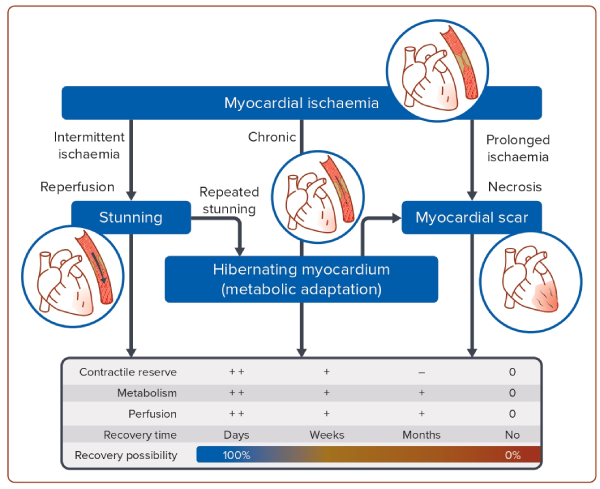
Ischaemic cardiomyopathy (ICM) is the most common cause of heart failure with reduced ejection fraction (HFrEF) and the largest contributing factor of the projected increases in heart failure (HF) incidence worldwide.1,2 The mechanism by which ICM leads to HF is through the development of left ventricular (LV) systolic dysfunction either via acute MI(s) or gradually via a progressive decline in systolic function without recognisable episodes of acute coronary syndromes (Figure 1). Pathophysiologically, the decline in LV contractile function is caused by a combination of scar tissue and areas of dysfunctional but still viable myocardium (stunning or hibernating myocardium).3
Patients with HFrEF (EF <35–40%) and ICM have a poor prognosis.4,5 European Society of Cardiology (ESC) guidelines recommend myocardial revascularisation using either coronary artery bypass grafting (CABG) or percutaneous coronary intervention (PCI). The rationale for coronary revascularisation in ICM is derived from the concept of reducing chronic ischaemia and restoring the function of hibernating myocardium.6 The prognostic and symptomatic benefits of myocardial revascularisation critically depend on the completeness of revascularisation.7 By ensuring normal coronary blood flow, the underlying aim is to restore and save myocardial tissue that has been damaged by ischaemia but is still vital from irreversible destruction via improved coronary blood flow (reversal of myocardial hibernation or stunning). This should eventually lead to an improvement in contractile cardiac function and ultimately in overall prognosis, however, the data are inconsistent.
This review aims to present current data on myocardial revascularisation strategies in HFrEF patients with ICM, the value of viability and ischaemia assessment prior to revascularisation, and explore the practical implications.
Revascularisation Strategies: CABG or PCI versus Optimal Medical Treatment
Evidence on the role of myocardial revascularisation in HFrEF stems largely from surgical investigations predating the pharmacological advances in HF treatment. In the CASS registry, patients with coronary artery disease (CAD) and severe LV systolic dysfunction without any systematic assessment of ischaemia or viability had a better prognosis when revascularised with CABG compared to medical treatment.8 Contemporary evidence on the role of revascularisation in HFrEF patients with ICM is derived from a limited number of randomised controlled trials (RCTs), namely STICH, PARR-2, HEART and REVIVED-BCIS2 (Table 1).9–14
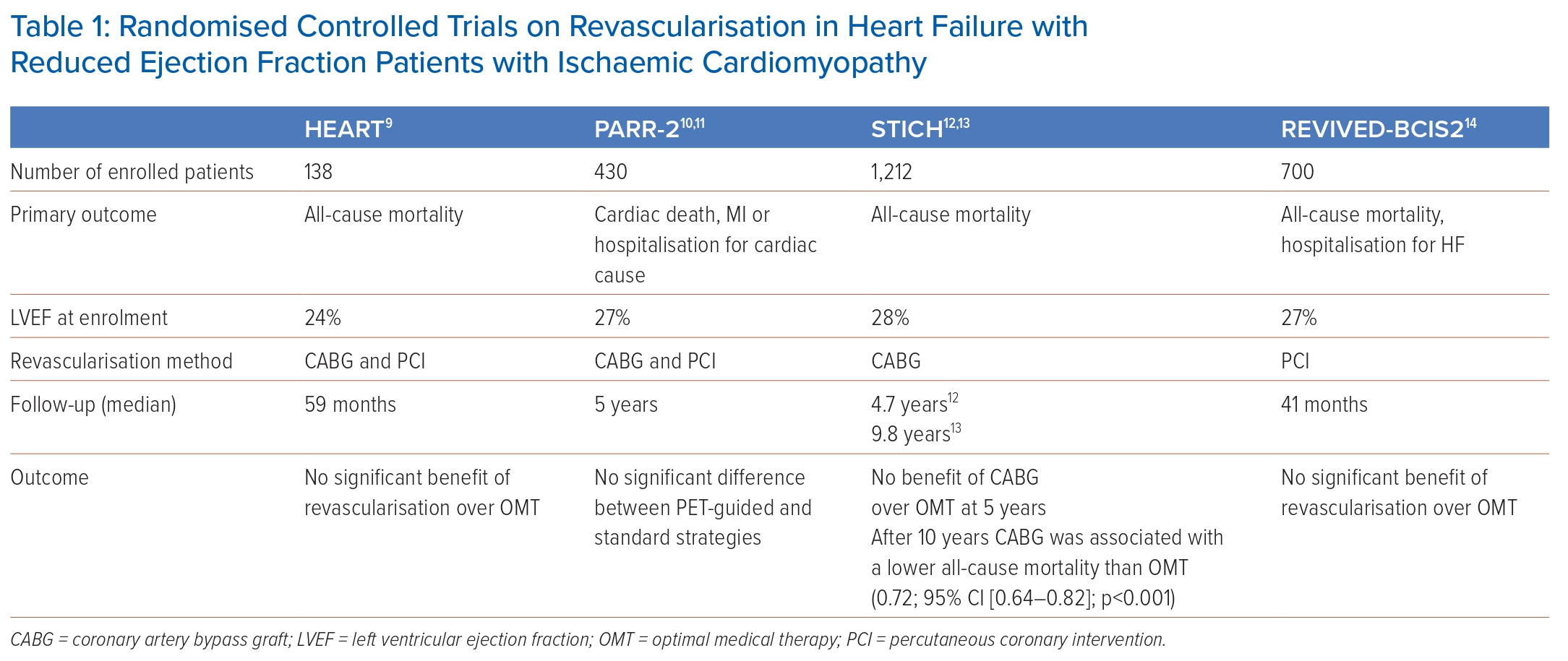
The STICH trial randomised 1,012 patients with extensive coronary artery disease and HFrEF (EF <35%) to either surgical revascularisation (CABG) on top of optimal medical treatment (OMT) or medical treatment alone. After a median follow-up of 56 months, no significant difference between the two arms with respect to the primary endpoint of death from any cause was found even though patients assigned to CABG had lower rates of death from cardiovascular causes and of death from any cause or hospitalisation for cardiovascular causes.12 However, Kaplan-Meier curves depicting the accrual of events – death from any cause or death from cardiovascular cause – over time crossed over at about 2 years, with a signal for a lower event rate in the CABG arm with long-term follow-up.12 In fact, long-term results of STICH (median follow-up duration: 9.8 years) demonstrated a 16% lower all-cause mortality in the CABG group compared to the medical treatment group, particularly in patients with coronary three-vessel disease or severely remodelled LV architecture (end-systolic volume index >78 ml/m2 or EF ≤27%).13 A moderate-to-severe mitral regurgitation (MR) was present in 18% of the patients in the STICH trial and adding mitral valve repair to CABG in patients with LV dysfunction and moderate-to-severe MR appeared to improve survival rates compared to CABG alone or medical therapy alone over a median follow-up period of 56 months (overall death rate 43%; HR versus OMT, 0.68; 95% CI [0.41–1.12]), whereas those undergoing isolated CABG did not (HR versus OMT, 1.20; 95% CI [0.77–1.87]).15
The PARR-2 trial included 430 patients with ICM and HFrEF (EF <35%) randomised to either imaging-guided management or standard care.11 Patients randomised to the imaging strategy underwent cardiac positron emission tomography (PET) for the evaluation of myocardial viability and prediction of the likelihood of the recovery of LV function following revascularisation. Invasive management was mandated in patients with high-to-moderate likelihood of recovery after myocardial revascularisation and discouraged in those with a low likelihood of recovery. The primary outcome was defined as cardiac death, MI, or recurrent hospital stays for cardiac causes at 1 year. Overall, the study could not demonstrate significantly fewer cardiac events in patients randomised to the PET-guided management versus standard care (RR 0.82; 95% CI [0.59–1.14]; p=0.16). However, approximately 25% of patients with PET-indicated revascularisation did not receive the intervention indicating a lack of adherence to the study protocol. Thus, the study results are substantially influenced by the deciding physician who chose to adhere to treatment indicated by PET based on clinical judgment and/or bias against revascularisation.
The HEART trial was originally designed to enrol 800 patients with ICM, HFrEF (<35%) and a significant burden of residual myocardial viability on pre-enrolment imaging.9 Patients were subsequently randomised to either optimal medical management or an invasive management. The study was stopped prematurely because of slow recruitment and only 138 patients were randomised. The cohort was largely underpowered, and no significant difference was detected between patients randomised to either management strategies.
Because of the early increase in periprocedural mortality and stroke observed with CABG, it was hypothesised that PCI would offer a better risk-benefit balance in patients with CAD with suitable coronary anatomy. On the other hand, PCI focuses on stenoses judged as haemodynamically significant and is associated with much less complete revascularisation when compared to CABG. Coronary pressure-derived fractional flow reserve (FFR) is the current standard of care for the functional assessment of lesion severity in patients with intermediate-grade stenosis (typically about 40–90% of all stenosis) without evidence of ischaemia in non-invasive testing, or in those with multivessel disease.7 The FAME trial showed that in patients with coronary multivessel disease randomised to an FFR-guided PCI strategy (using a cut-off ≤0.80 to indicate requirement for PCI), long-term outcomes in terms of death or non-fatal MI were superior compared with angiography-guided PCI.16–18 A large meta-analysis of 9,173 lesions could further demonstrate that with lesions with FFR <0.75, revascularisation reduced the 1-year composite risk of death and MI.19
Results of the Swedish Coronary Angiography and Angioplasty Registry suggested that CABG was associated with lower mortality rates than PCI.20 The aim of the REVIVED-BCIS2 trial was to investigate whether revascularisation using PCI in addition to optimised drug therapy was superior to optimised drug therapy alone regarding mortality and hospitalisation for HF.14 For this purpose, the REVIVED-BCIS2 trial randomised 700 patients with ICM and a confirmed EF ≤35%. No significant difference in the occurrence of the primary endpoints was seen over a median observation period of approximately 3.5 years between the PCI or optimal drug therapy group (HR 0.99; 95% CI [0.78–1.27]; p=0.96) and the EF did not improve in the PCI group. On the other hand, the REVIVED-BCIS2 trial detected no increase in periprocedural mortality compared to the STICH trial. The results from the REVIVED-BCIS2 trial thus differ from the short-term results of the STICH trial, in which bypass surgery was associated with an increased risk of mortality.12 A longer follow-up period will be required to reach a reliable conclusion.
Regardless of the relatively short observation period, the REVIVED-BCIS2 study raised important methodological questions. During the trial, the extent of CAD and possible myocardial ischaemia were not adequately considered prior to revascularisation. About 50% of patients had only two-vessel CAD which suggests a less severely affected group of patients compared to those in the STITCH trial. Moreover, data on the degree of stenosis and functional measurements on the haemodynamic significance of the existing stenoses – indicated by FFR measurements – are lacking. REVIVED-BCIS2 was mainly composed of patients with New York Heart Association (NYHA) I and II symptoms with little or no angina, while patients in STICH were more symptomatic with 86% of the CABG arm and 85% of the OMT arm reporting NYHA functional class II or III symptoms, and 43% of both arms reporting Canadian Cardiovascular Society class II symptoms for angina.
Additional key differences between REVIVED-BCIS2 and STICH are found in the patient populations and the underlying medical treatment. The REVIVED-BCIS2 trial consisted of an older patient population with a mean age of 70 and 69 years in the PCI and OMT groups, respectively, while the mean age in the STICH trial was 60 and 59 years in the CABG and OMT groups, respectively.12,14 When STICH was recruiting in 2002 to 2007, the only class I recommended medical therapies for HFrEF were angiotensin-converting enzyme inhibitors (ACE-I), ß-blockers and digoxin.21 When REVIVED-BCIS2 started recruiting in 2013, angiotensin II receptor blockers (ARB), mineralocorticoid receptor antagonists (MRA), hydralazine and isosorbide dinitrate had been added as new class I recommendations.22 This was followed by the addition of angiotensin receptor/neprilysin inhibitors (ARNI) and sodium–glucose co-transporter 2 inhibitors (SGLT-2i) as class I recommendations.23 Unsurprisingly, significant differences in medical therapy can be seen with ACE-I employed in 84% versus 80%; ß-blockers in 83% versus 88%; and digoxin in 20% versus 21% of the CABG and OMT arms of STICH, respectively. ACE-I were used in 61% versus 57%, angiotensin receptor blockers (ARB) in 17% and 19%, ARNI in 26% and 33%, MRA in 49% and 57%, ß-blockers in 93% and 94%, and oral hypoglycaemic agents in 28% and 29% in the PCI versus OMT arms of REVIVED-BCIS2, respectively. Finally, as the STITCH trial was conducted earlier when different guidelines were in place, devices including CRT and ICDs, which are now considered a critical part of OMT, were more frequently used in patients in the REVIVED-BCIS2 compared with those in the STICH trial.11,14
Viability and Ischaemia Testing Prior to Revascularisation
Although earlier retrospective observational studies and several meta-analyses suggested that viable myocardium has the potential to recover its function as a consequence of revascularisation, several significant limitations precluded the acceptance of these data as conclusive evidence.24–28 Even more recent evidence stems from several RCTs, but again the individual results are inconclusive.
In the STICH trial, no significant interaction was found between myocardial viability and benefit from CABG over medical treatment alone, relating to clinical outcomes and post-revascularisation improvements in LVEF.13,29,30 On the other hand, viability was not assessed with contemporary and accurate imaging modalities, such as cardiac MRI or PET. Testing for inducible ischaemia prior to surgery also did not modify outcomes for revascularisation.31
In the PARR-2 study, PET-guided revascularisation was mandated only in patients with a significant burden of hibernating myocardium. Results showed a similar rate for the composite endpoint of cardiac death, MI or cardiac hospitalisation in the two arms [RR 0.82; 95% CI [0.62–1.07]; p=0.15] compared to standard care.10,11 Again, these results warrant careful interpretation due to a lack of adherence to the study protocol in a substantial number of study participants resulting in low statistical power. When a post-hoc analysis was restricted to patients whose management adhered to PET recommendations, there was a significant benefit of PET-guided management over standard care (RR 0.73; 95% CI [0.54–0.99]; p=0.042), though the difference was largely driven by cardiac rehospitalisation, but not mortality.32
In the HEART trial, participants were screened for viable myocardium via dobutamine stress echocardiography.9 An inclusionary prerequisite was the presence of at least five viable LV segments with reduced contractility using a 17-segment model. The primary outcome revealed non-inferiority of medical therapy. This study was, however, underpowered secondary to a relatively small sample size. Furthermore, the primary modality of viability assessment was dobutamine stress echocardiography which has a lower sensitivity for viability detection relative to other imaging modalities. Additionally, randomisation had not occurred prior to viability assessment, therefore clouding the impact of viability assessment on treatment outcomes.
The REVIVED-BCIS-2 trial undertook contemporary myocardial viability testing with cardiovascular MRI or stress echocardiography prior to revascularisation.33 Evidence of myocardial viability did not identify a population of patients who benefited from PCI. The extent of non-viable myocardium was associated with a higher risk of death or hospitalisation for HF and a lower chance of improvement in LV function. Findings suggest that the extent of dysfunctional, yet viable, myocardium was not associated with revascularisation outcomes.33
The ISCHEMIA trial included 5,179 participants with at least moderate ischaemia on non-invasive stress testing and were randomised to a routine invasive strategy or conservative strategy with angiography reserved for failure of medical therapy. Individuals with an EF <35% by any imaging modality, NYHA class III or IV, severe symptoms of angina on medical therapy at baseline, or PCI or CABG within 1 year before enrolment were excluded. No benefit or treatment interaction between the severity of ischaemia and improved outcomes with revascularisation was detected.34 A sub-analysis of the trial suggested a possible benefit of coronary revascularisation in patients with HF and mild-to-moderately reduced EF (35–45%). However, these positive results of revascularisation were driven by a small study sample (n=28) and generalisability is limited.35
A recent meta-analysis including data from the STICH, HEART, PARR-2 and REVIVED-BCIS-2 trials investigated the effect of viability-guided management on all-cause and cardiac mortality.36 No difference in the event rate was observed in ICM patients who underwent revascularisation following the results of viability assessment versus those who were considered non-adherent. However, despite the lack of evidence on any prognostic advantage of viability-guided revascularisation, these results have to be interpreted with caution as the hypothesis that dysfunctional but viable myocardium may recover with revascularisation may still be valid.3 Specifically, it has to be determined prior to myocardial revascularisation if there is a concordance with viable myocardial segments and vessels suitable for revascularisation. Recent in-depth analyses suggest that patients ‘without viability’ require a more individualised approach and myocardial viability assessment remains an important part of the evaluation of ICM.3
Critical Appraisal
The prognosis of HFrEF patients is largely dominated by LV systolic dysfunction. Over the past few years, remarkable advances in medical HF treatment such as sacubitril-valsartan and SGLT2 inhibitors have been made that resulted in a substantial improvement of prognosis.37–40 Moreover, cardiac resynchronisation therapy (CRT) combined with an ICD reduced the risk of death and HF events in HFrEF patients with wide QRS complex.41,42 Additionally, the COAPT trial could show that in symptomatic HF patients with severe mitral regurgitation transcatheter mitral valve approximation using the MitraClip on a background of maximally tolerated guideline-directed medical therapy was superior to medial therapy alone in reducing HF hospitalisation and mortality.43,44
The role of myocardial revascularisation in ICM with HFrEF still remains controversial.36 Evidence prior to the latest medical treatment advances suggests there is a benefit of having CABG over pharmacological therapy in patients with severe CAD and HFrEF, although the benefit may only become apparent during long-term follow-up.12,13 PCI-based revascularisation in ICM patients has a rationale when complete revascularisation can be expected, but lacks conclusive evidence. Data from the Swedish Coronary Angiography and Angioplasty Registry indicate that PCI has become the revascularisation strategy of choice in ICM with almost three times more procedures than CABG.20,36 Results from the REVIVED-BCIS2 trial do not support the widespread use of PCI-based revascularisation in ICM but the trial suffers from several significant limitations.14 PCI appears to have a role in patients with angina-related symptoms under OMT and ineligibility to CABG after careful consideration of perioperative risk.14
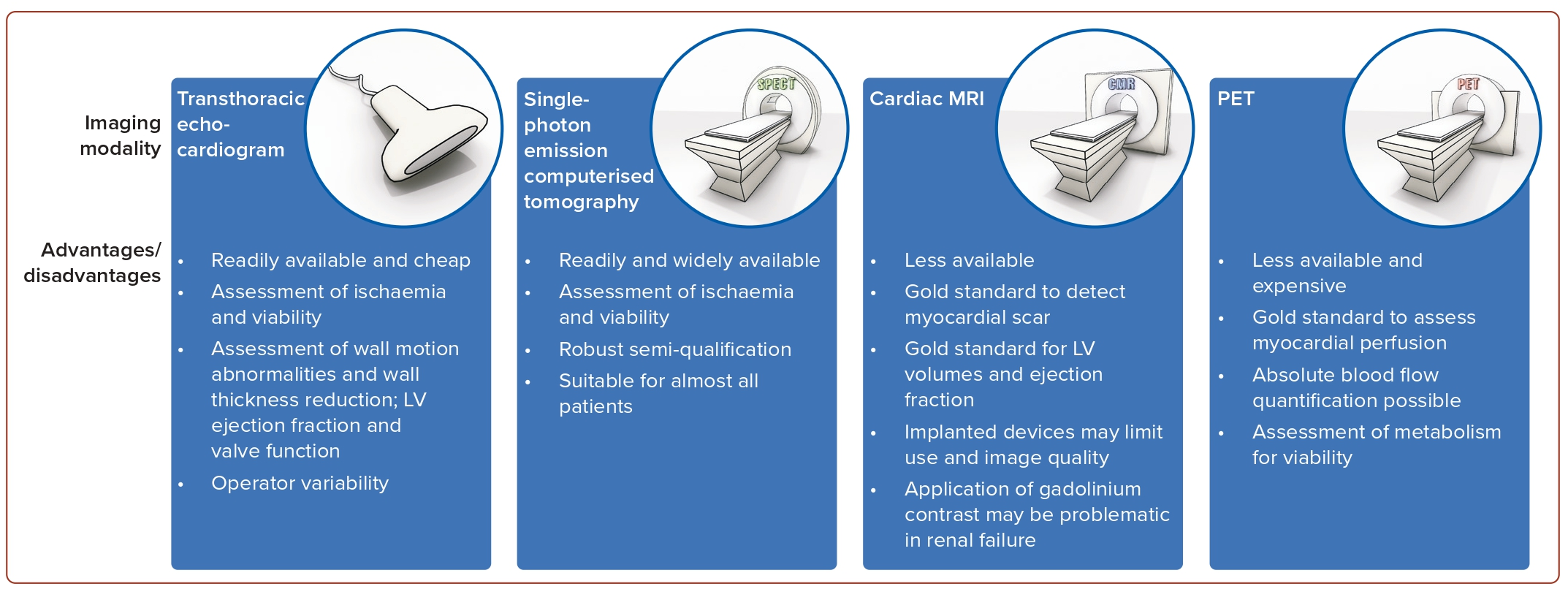
Based on current data it is not mandatory to use the extent of myocardial viability or the extent of inducible ischaemia to gauge the individual patient’s benefit from myocardial revascularisation.36 Nonetheless, in patients with symptoms ranging between HF and angina, viability or ischaemia assessment can offer valuable guidance in decision-making to select patients that are more likely to benefit from myocardial revascularisation.3 Retrospective evidence in patients not selected for ejection fraction suggests a possible benefit of ischaemia-guided revascularisation in the presence of severe ischaemic burden (>10 to 15% of the LV myocardium).36,45 Several imaging modalities have been deemed appropriate for the assessment of ischaemia and viability (Figure 2). Myocardial contrast echocardiography, single-photon emission CT (SPECT) and late gadolinium enhancement cardiac magnetic resonance (LGE-CMR) all assess cellular integrity. PET assesses cellular metabolism, whereas dobutamine techniques assess contractile reserve. Current ESC guidelines state that non-invasive stress imaging (CMR, echocardiography, SPECT or PET) may be considered for the assessment of myocardial ischaemia and viability in patients with HF and CAD, who are suitable for coronary revascularisation, before making a decision about revascularisation.7
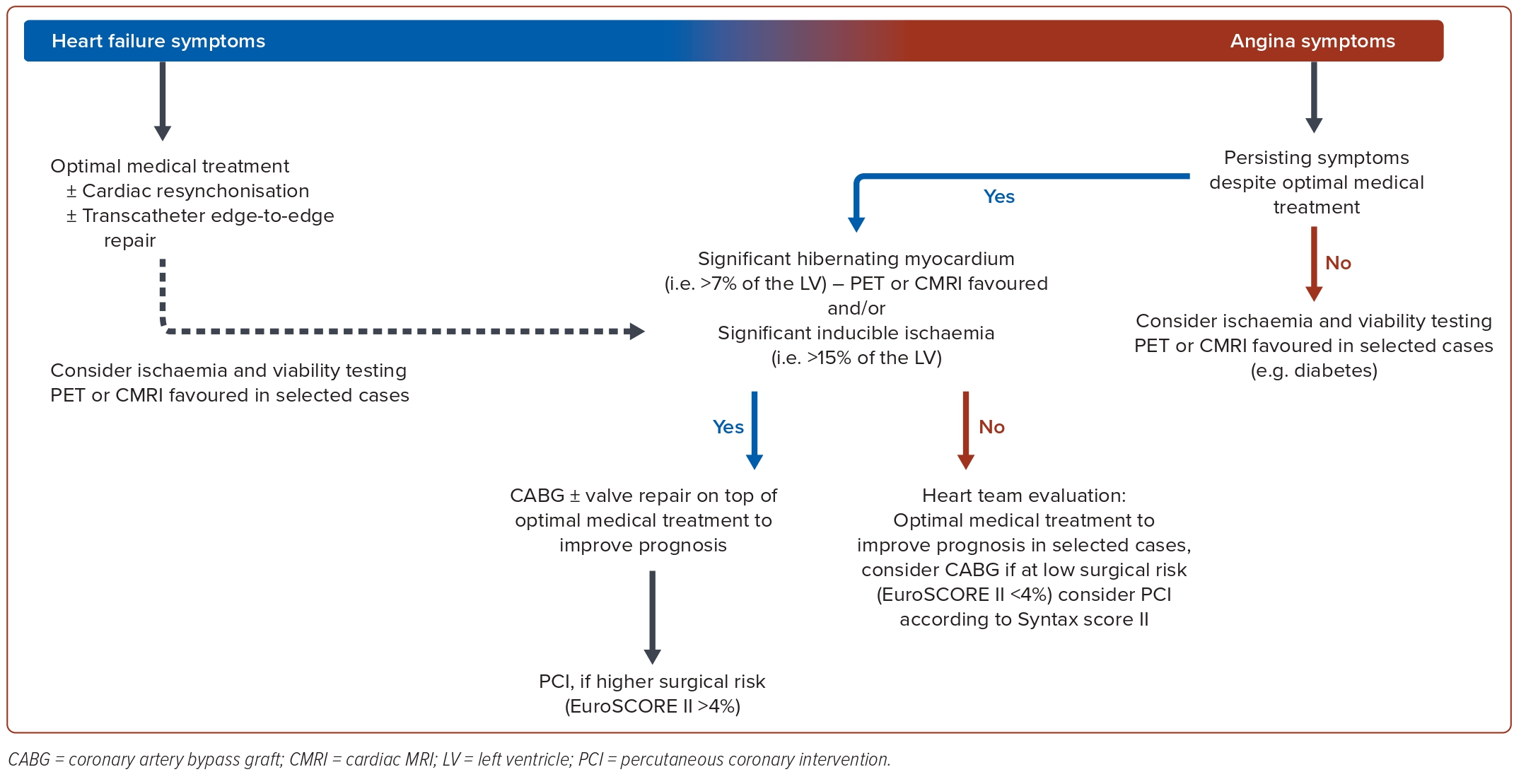
In light of the partly conflicting evidence, clinicians face the task of reconciling conflicting trial outcomes and the partly confusing findings related to myocardial viability. For the daily management of HFrEF patients with ICM, we suggest the following algorithm and synthesis of available major findings (Figure 3).36,46,47 The primary task for clinicians is to identify if the patients’ symptoms are more related to HF driven by myocardial dysfunction or related to angina.
In cases where HF symptoms dominate, OMT and assessment for a potential use of CRT therapy and/or transcatheter mitral valve repair seems prudent. In cases where angina symptoms dominate despite OMT, the extent of myocardial ischaemia should be determined. In the presence of severe inducible ischaemia (>10–15% of LV mass), surgical revascularisation in addition to valve repair and OMT is advised to improve prognosis. As an alternative to CABG, PCI has a role in high-risk surgical situations and in case of coronary lesions with lower complexity.14,34 Coronary revascularisation also remains an option in the presence of limited myocardial ischaemic burden and angina symptoms that are refractory to medical treatment. Again, available evidence supports CABG as the primary revascularisation strategy and ischaemia-guided PCI is reserved to high-risk surgical patients or in case of lower coronary complexity.31,48
When no typical angina symptoms are present or in selected cases of patients with HF, such as those with diabetes, an assessment of myocardial viability and ischaemia testing can be useful to evaluate the extent of ischaemia, myocardial scar and viable hibernating myocardium for the consideration of potential myocardial revascularisation strategies. A decision-making threshold for the extent of hibernating myocardium may be set at around 7% of LV mass beyond which one could possibly favour revascularisation.49
Regarding the revascularisation modality, available evidence points to CABG being the best way to achieve complete coronary revascularisation.12,13 However, an evaluation of surgical risk and coronary complexity is always mandatory for final decision-making. The Society of Thoracic Surgeons (STS) risk score and EuroSCOREII are suitable to predict the 30-day postoperative mortality risk of CABG.50 A 30-day perioperative mortality <4% appears to be acceptable to prompt surgery. In patients deemed at higher risk for CABG (30-day perioperative mortality >4%), the SYNTAX score/SYNTAX score II is a useful tool for considering PCI by assessing coronary complexity.51 PCI can be considered in patients with lower coronary complexity (SYNTAX score <22).7 Diabetes may mask the presence of angina. In these patients, coronary revascularisation with CABG if feasible and with acceptable perioperative leads to lower all-cause mortality than with PCI in long-term follow-up due to the expected higher rate of complete myocardial revascularisation.52,53
Whether the OMT for HFrEF should include statin therapy is unresolved. Observational data suggest safety and benefit, however, inconclusive evidence stems from two RCTs: CORONA and GISSI-HF.54–56 A 2019 systematic review of 17 studies, including two RCTs and 15 prospective cohort studies comprising 88,100 patients, reported a beneficial effect on cardiovascular outcomes irrespective of the aetiology of HF and EF level.57 Recent large real-world observational findings also supports a potential benefit of using statins for HFrEF.58 ESC guidelines indicate that the routine use of statins in patients with HF without other indications for their use, such as CAD, is not recommended but it is recommended for individuals who have a high risk for atherosclerotic cardiovascular disease to reduce HF hospitalisations.39 The American College of Cardiology/American Heart Association cholesterol management guideline gives use of statins a IIb class of recommendation (with usefulness/efficacy less well established by evidence/opinion) for ICM with HFrEF and a reasonable life expectancy.59
Conclusion
The overall aim of treating CAD is to slow disease progression and to reduce thrombotic complications. This can be achieved by modifying lifestyle and risk factors. The role and significance of revascularisation in patients with stable CAD has largely been reduced by the recent progress in pharmacotherapy.34,60 Coronary revascularisation is largely confined to symptom control in patients refractory to adequate medical management or to interventions in the presence of significant ischaemia or hibernating myocardium. OMT is crucial since even in the ISCHEMIA trial only 40% of the participants received OMT.34 Current efforts and resources should be directed to ensure optimal HF treatment for patients with ICM with HFrEF as the first and most important measure to improve long-term prognosis and to then focus on treatments related to specific symptoms. 
Clinical Perspective
- In patients with heart failure with reduce ejection fraction and ischaemic cardiomyopathy, the primary task for clinicians is to identify if symptoms are more related to HF driven by myocardial dysfunction or related to angina.
- In cases where HF symptoms dominate, optimal medical therapy (OMT) and assessment for possible cardiac resynchronisation therapy and/or transcatheter mitral valve repair is advisable.
- In cases where angina symptoms dominate despite OMT, the extent of myocardial ischaemia should be determined. In the presence of severe inducible ischaemia, surgical revascularisation with coronary artery bypass graft (CABG) in addition to valve repair and OMT is recommended to improve the patient’s prognosis. Percutaneous coronary intervention has a role as an alternative to CABG in patients who have a high surgical risk and in case of coronary lesions with lower complexity.