Coronary artery calcium (CAC) is an established marker of subclinical atherosclerosis and an independent predictor of future coronary artery disease (CAD).1,2 The CAC score, determined using non-contrast coronary CT (CCT), can assist in cardiovascular (CV) risk assessment and clinical decision-making.3 The presence and extent of CAC are associated with an increased risk of CV events with an incremental value beyond traditional CV risk factors.4 Vascular calcification is an active and regulated process integral to CV disease and is intimately linked to hypertension.5 A study by Grossman et al. showed that the presence of CAC in normotensive healthy subjects can predict the development of hypertension.6
Left ventricular diastolic dysfunction (LVDD) is an alteration of left ventricular (LV) diastolic filling due to decreased myocardial relaxation and increased LV stiffness. Assessment of LV diastolic function is important since most patients with heart failure demonstrate an abnormal diastolic filling pattern due to LVDD. LVDD is common in patients with hypertension and is a predictor of increased adverse CV outcomes.7 Previous studies have revealed the incidence of LVDD between 20–58% in hypertension.8–10 The mechanisms, including increased afterload, myocardial fibrosis and inflammation, would cause LVDD in patients with hypertension.11,12
Given CAC score and LVDD are strong predictors of CV events and share common risk factors, previous studies have shown a relationship between LVDD and CAC score in several patient populations, including asymptomatic patients, elderly patients and patients with diabetes.13–15 However, there is no specific study regarding an association between CAC score and LVDD in patients with hypertension. We hypothesise that patients with hypertension could have a relationship between CAC score and LVDD due to an atherogenic process and inflammation. This study aimed to investigate the association between CAC and LVDD in patients with hypertension.
Methods
Study Population
Consecutive patients aged 18 years or older with hypertension who underwent both non-contrast CCT for CAC scoring and resting echocardiography between 2016 and 2020 in a tertiary hospital were studied. Hypertension was defined as a self-reported history of hypertension, the use of antihypertensive medication or a blood pressure (BP) of ≥140/90 mmHg. The grading of hypertension was defined in accordance with the latest European guideline for hypertension. Grade 1 hypertension was classified as a systolic BP of 140–159 mmHg and/or a diastolic BP of 90–99 mmHg. Grade 2 hypertension was characterised by a systolic BP of 160–179 mmHg and/or a diastolic BP of 100–109 mmHg. Grade 3 hypertension was defined as a systolic BP of ≥180 mmHg and/or a diastolic BP of ≥110 mmHg.16 Patients with a history of CAD or MI, prior cardiac surgery or coronary revascularisation, AF, severe left-sided valvular disease, any prosthetic cardiac valve, LV systolic dysfunction with left ventricular ejection fraction (LVEF) of <50%, congenital heart disease, permanent pacemaker, limited or poor-quality echocardiography or incomplete data were excluded.
The protocol for this study was approved by the institutional review board. The requirement to obtain written informed consent was waived due to the retrospective design of the study.
Clinical symptoms, CAD risk factors and details of current medications were obtained from electronic medical records. Diabetes was defined as a self-reported history and/or receiving treatment for diabetes, or a fasting glucose of ≥126 mg/dl. Dyslipidaemia was defined as a total cholesterol level of ≥240 mg/dl, an LDL cholesterol level of ≥130 mg/dl, an HDL cholesterol level of <40 mg/dl, a triglyceride level of ≥200 mg/dl, and/or treatment with a lipid-lowering agent. BP and heart rate data were obtained before CCT. Laboratory results, including serum creatinine, estimated glomerular filtration rate (eGFR), haematocrit, fasting plasma glucose, total cholesterol, HDL cholesterol, LDL cholesterol and triglyceride were obtained from the medical records within 3 months of CCT. The eGFR was calculated using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation.17
CCT Protocols and Echocardiography
CCT was performed using a 256-slice scanner (SOMATOM Definition Flash, Siemens Healthcare) according to established guidelines and institutional protocol at the time of the scan.18 A non-enhanced prospective electrocardiography (ECG)-gated sequential CCT scan was performed to determine CAC scoring using the following parameters: rotation time of 280 ms, slice collimation of 0.6 mm, slice width of 3.0 mm, tube voltage of 120 kV and tube current of 50 mAs. Analysis of CAC images was performed following the standard protocol on a separate workstation (syngo.via, Siemens Healthineers). CAC scans were interpreted according to the Agatston method with a high CAC score defined as greater than median CAC score.19
Echocardiograms were performed by experienced cardiologists. A comprehensive transthoracic echocardiographic examination consisted of 2D, M-mode, Doppler and tissue Doppler imaging (TDI). All echocardiographic measurements were performed according to the standard guidelines with the average of 3–5 consecutive cardiac cycles for the analyses.20 Diastolic function was assessed by pulsed-wave Doppler examination of mitral flow and TDI of the mitral annulus.21 Peak early (E) and late (A) diastolic velocities of mitral inflow and E-wave deceleration time were measured using pulsed-wave Doppler study with the sample volume at the tip of mitral valve. Longitudinal early (e’) and late diastolic myocardial velocities were measured using TDI in apical four-chamber view with the sample volume at the medial and lateral aspects of mitral annulus. The average of medial and lateral e’ was used for the E/e’ ratio.21 Left atrial (LA) volume and LV mass were measured using the recommendations by the American Society of Echocardiography and indexed with body surface area.20 LVDD was diagnosed if three or more of the following parameters met the cut-off values: septal e’ <7 cm/s or lateral e’ <10 cm/s, average E/e’ ratio > 14, left atrial volume index (LAVI) >34 ml/m2 and peak tricuspid regurgitation velocity >2.8 m/s.21
Statistical Analysis
All statistical analyses were performed using SPSS Statistics for Windows version 20.0 (SPSS Inc). Continuous variables with normal distribution were presented as mean (±SD) and continuous variables with non-normal distribution were presented as median and interquartile ranges (IQR). The normality of the distribution of variables was examined by the Kolmogorov-Smirnov test. Categorical variables were present as absolute numbers and percentages. Differences between patient characteristics with and without LVDD as well as high (>median CAC) and non-high CAC (≤median CAC) in terms of baseline and image characteristics were compared using the Student’s unpaired t-test or the Mann-Whitney U-test for continuous variables, while the χ2-square test or Fisher’s exact test was used for categorical variables as appropriate. Normally distributed continuous data of multiple (>2) groups were compared using one-way analysis of variance. Non-normally distributed continuous data of multiple (>2) groups were compared using the Kruskal–Wallis test. Univariable Cox logistic regression analysis was performed to identify significant predictors of LVDD and high CAC. Variables with a p value <0.05 from univariable analysis were entered into Cox logistic regression multivariable analysis. The results of the univariable and multivariable analyses are given as odds ratios along with their respective 95% CI. A p-value <0.05 was considered statistically significant for all tests.
Sample size estimation was performed based on a study by Osawa et al. that examined the association between CAC and LVDD in elderly people.15 The median CAC of patients with LVDD was 91 (IQR 455), while the median CAC of patients without LVDD was 25 (IQR 186). The sample size was determined using the Mann–Whitney test, with a type I error rate of 5% and a test power of 80% to detect an estimated standard deviation from the 10th percentile. Therefore, the total number of patients needed was 242.
Results
Study Population
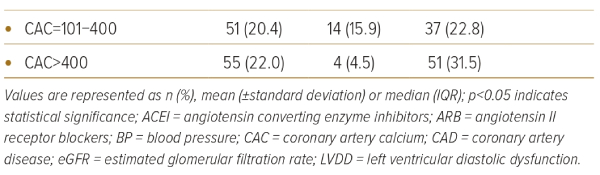
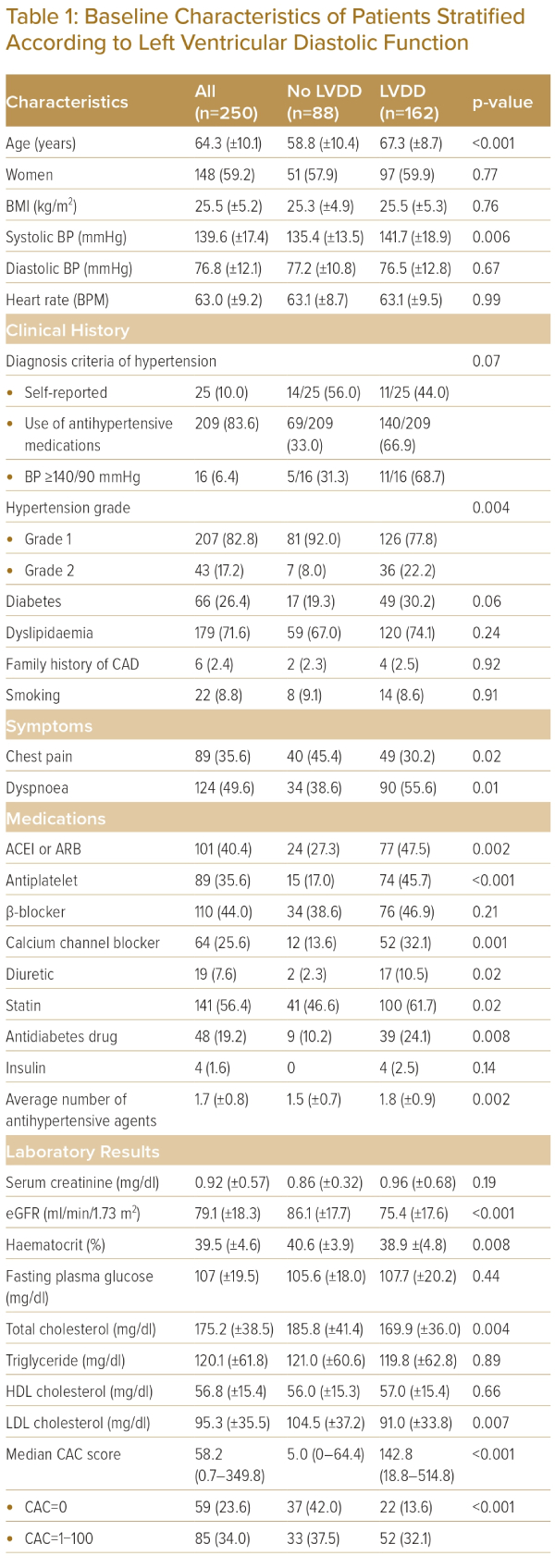
There were 250 patients with hypertension included in the analyses. Table 1 shows baseline characteristics, laboratory data, and CAC scores of all patients as well as the comparisons between patients with and without LVDD. The common primary indications for echocardiography and CCT were dyspnoea (49%) and chest pain (35%). Mean age was 64.3 (±10.1) years (59% women). Eighty-two percent of patients had grade 1 hypertension. The rest had grade 2 hypertension. The average number of antihypertensive drugs was 1.7 (±0.8). LVDD was present in 162 (64%) patients. The median CAC score was 58.2 (IQR 0.7, 349.8) and 191 (76%) patients had a CAC score >0.
Regarding the inclusion criteria, 25 patients reported having hypertension, 209 patients were using antihypertensive medications and 16 patients had a BP of ≥140/90 mmHg without taking medications. There were no significant differences observed in the prevalence of LVDD among these categories. Specifically, the prevalence of LVDD was 44.0% (11 of 25) in the self-report group, 66.9% (140 of 209) in the antihypertensive medication group and 68.7% (11 of 16) in the group with BP ≥140/90 mmHg (p=0.07) (Table 1). Additionally, there were no significant differences in CAC scores between these groups. The median CAC scores were as follows: self-reported group 18.1 (IQR 0, 105.5), hypertensive medication group 69 (IQR 1.9, 379.5), and BP ≥140/90 mmHg group 27.2 (IQR 1.1, 194.9) (p=0.06).
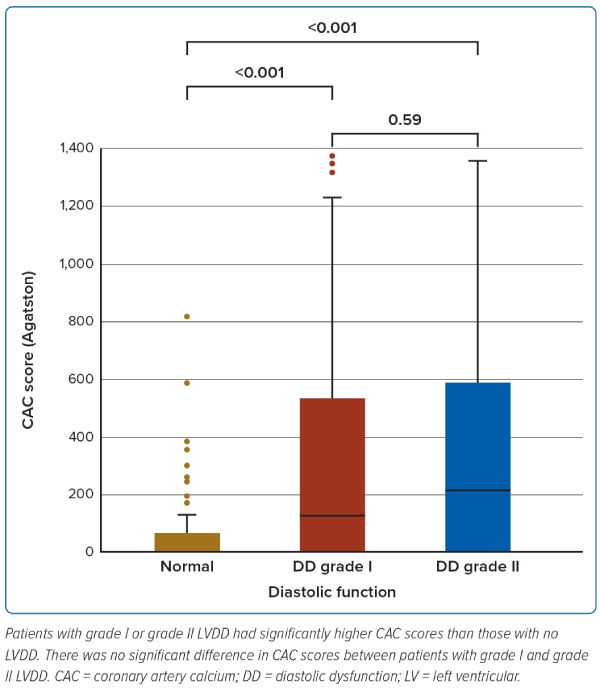
Patients with LVDD were older (67.3 ± 8.7 versus 58.8 ± 10.4; p<0.001) and likely to present with dyspnoea (55.6% versus 38.6%; p=0.01). Compared to those without LVDD, they had a higher severity of hypertension, with higher systolic blood pressure (141.7 ± 18.9 mmHg versus 135.4 ± 13.5 mmHg; p=0.006), higher prevalence of grade 2 hypertension (22.2% versus 8%; p=0.004) and used a greater number of antihypertensive medications (1.8 ± 0.9 versus 1.5 ± 0.7; p=0.002) . Patients with LVDD had higher median CAC scores (142 [IQR 18.8; 514.8] versus 5.0 [IQR 0; 64.4], p<0.001). Figure 1 shows that CAC scores of patients with grade I or grade II LVDD were significantly higher than patients without LVDD (median CAC score; grade I LVDD=127.3 [IQR 17.9; 509.6], grade II LVDD=215.3 [IQR 21.0; 532.3]). However, there was no significant difference in median CAC scores between patients with grade I and grade II LVDD (p=0.59).
Echocardiographic Data
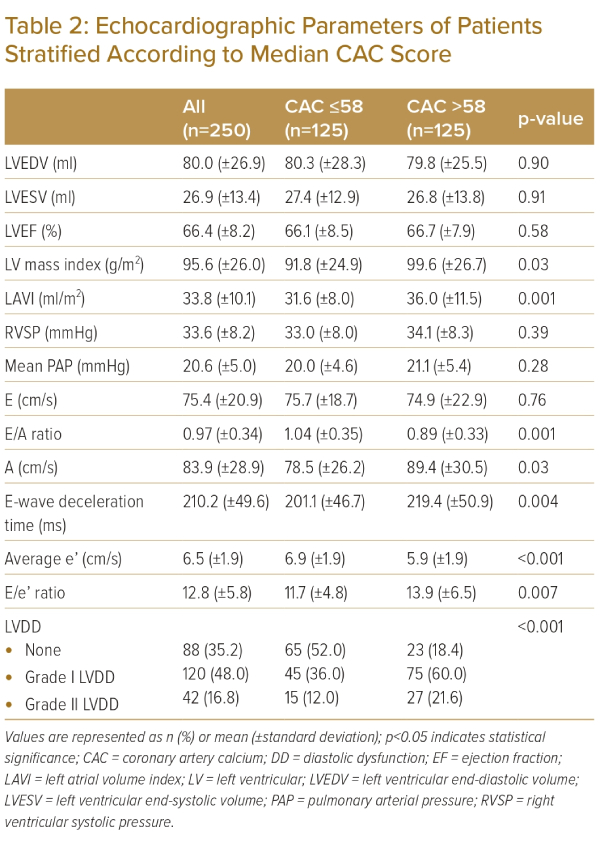
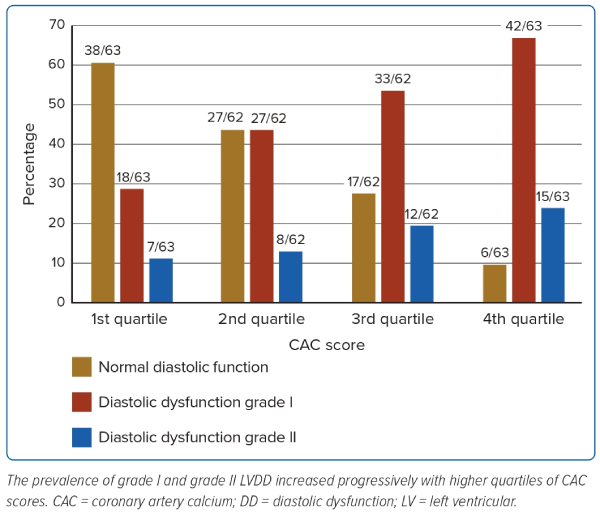
Table 2 demonstrates echocardiographic data in all patients as well as the comparisons between patients with and without high CAC scores (>median CAC score of all patients; 58.2). The average LVEF was 66.4% (±8.2) and 48% of patients had grade I diastolic dysfunction and 16.8% had grade II. Patients with high CAC had higher LV mass index, LAVI and greater prevalence of diastolic dysfunction as well as greater severity of diastolic dysfunction. Figure 2 shows diastolic function categories stratified by quartiles of CAC scores. The prevalence of grade I and grade II LVDD progressively increased according to quartiles of CAC scores. Patients with the fourth quartile of CAC score had over double the rates of grade I and grade II LVDD compared to the first quartile. Patients with the first quartile had the highest rate of normal diastolic function (60.3%).
Multivariable Analyses
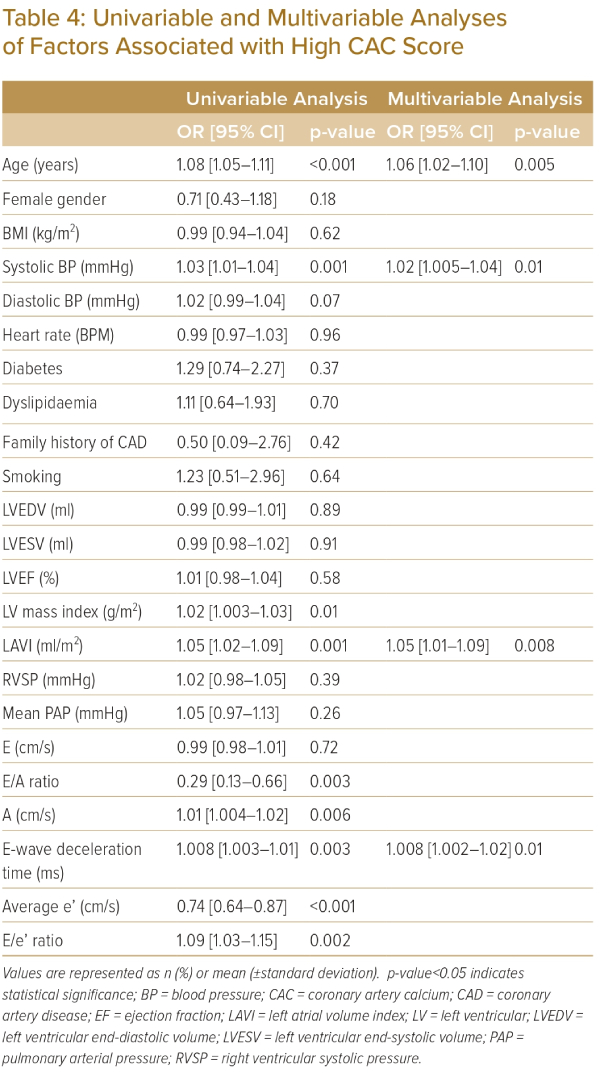
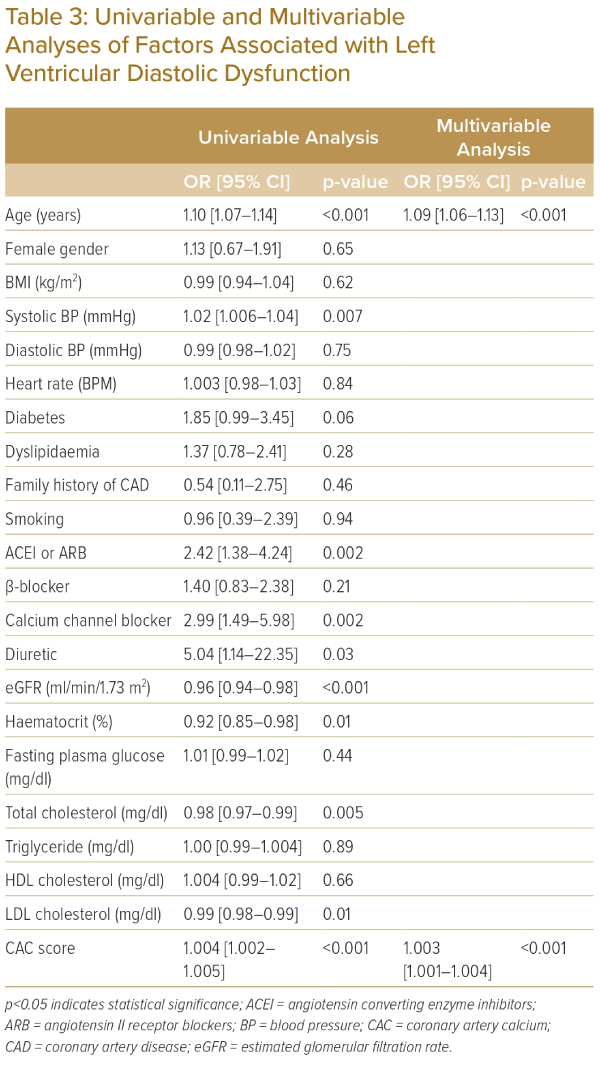
Table 3 shows the association between LVDD and clinical variables and CAC score. The univariable analysis indicates that age, systolic BP, the use of angiotensin-converting enzyme inhibitors or angiotensin-receptor blockers, calcium channel blockers, diuretics, eGFR, hematocrit and levels of total and LDL cholesterol as well as CAC scores are associated with LVDD. Multivariable analysis demonstrated age (OR 1.09; 95% CI [1.06–1.13]; p<0.001) and CAC score (OR 1.003; 95% CI [1.001–1.004]; p<0.001) were independently associated with LVDD. Table 4 shows the association between high CAC (>58.2), clinical variables and echocardiographic parameters. Multivariable analysis demonstrated age (OR 1.06; 95% CI [1.02–1.10]; p=0.005), systolic BP (OR 1.02; 95% CI [1.005–1.04]; p=0.01), LAVI (OR 1.05; 95% CI [1.01–1.09]; p=0.008) and E-wave deceleration time (OR 1.008; 95% CI [1.002–1.02]; p=0.01) were independently associated with high CAC score.
Discussion
The study, conducted in patients with hypertension without CAD who underwent both echocardiography and non-contrast CCT, yielded several key findings. Firstly, it revealed that 64% of the patients had LVDD. Secondly, those with LVDD exhibited higher CAC scores compared to those without LVDD. Thirdly, CAC was independently associated with LVDD. Finally, LAVI and E-wave deceleration time were both independently associated with high CAC scores.
CAC is now established as a reliable tool for estimating the risk of MI, coronary death and all-cause mortality.2,22 Current guidelines endorse using non-contrast CCT to assess CAC in suitable asymptomatic patients to improve clinical risk evaluation.22,23 Previous studies showed CAC was associated with age, CAD risk factors such as hypertension, diabetes, dyslipidaemia and a family history of CAD.24,25 In our study, 76% of the patients had a CAC score over 0. The multivariable analysis showed age and systolic BP were independently associated with high CAC. These were consistent with previous studies.15,24,25
LVDD is associated with increased myocardial fibrosis and ventricular stiffness. Comorbidities associated with LVDD include hypertension, diabetes, obesity, CAD and smoking.26 LVDD has been recognised in several cardiovascular diseases and is associated with worse outcomes, including all-cause mortality.7,11,12,27 The mechanism underlying the association between high CAC scores and LVDD can be explained by several factors.
First, the presence of extensive coronary artery calcification indicates a higher burden of atherosclerosis and plaque formation within the coronary arteries. This can lead to reduced coronary blood flow and impaired perfusion of the myocardium, affecting the overall cardiac function, including diastolic function.28 Second, the accumulation of calcium deposits in the coronary arteries reflects the chronic inflammatory process associated with atherosclerosis. Inflammation can contribute to the development of LVDD by promoting myocardial fibrosis, impaired relaxation and increased stiffness of the LV. Furthermore, coronary artery calcification is closely associated with other cardiovascular risk factors such as hypertension, diabetes and dyslipidaemia. These risk factors can directly influence LVDD through their effects on myocardial remodelling, vascular function and neurohormonal imbalances.
In a study by Osawa et al. on elderly individuals, age, female sex and hypertension were found to be independently associated with LVDD.15 Haddad et al. conducted a large population-based study and found that advanced LVDD (>grade I) was independently associated with age, female sex, being black, lower height, higher body weight, higher pulse pressure, higher HbA1c, lower HDL-C and prevalent CAD.29 Both studies demonstrated that CAC was independently associated with LVDD.15,29 Our study also found that patients with LVDD were older and had a higher hypertension grade, which is consistent with previous studies that have shown an association between LVDD and age and CV risk factors.15,29 Furthermore, our study adds to the existing literature by demonstrating an association between CAC and LVDD in patients with hypertension.
Echocardiography is the main imaging modality for assessment of LV diastolic function. Multiple echocardiographic variables can be used to determine LV diastolic function. Like the previously published data, the present study confirms that patients with a high CAC score had worse diastolic parameters, such as higher A, E-wave deceleration time, E/e’ ratio, LV mass index and LAVI than those with lower CAC.15,29 Furthermore, LAVI and E-wave deceleration time were independently associated with a high CAC score representing the link between LVDD and CAC in patients with hypertension. LA volume is directly related to LV filling pressure and it has been shown to be a predictor of stroke and development of heart failure.27
Nearly half of the patients in this study reported experiencing dyspnoea, especially in patients with LVDD. It has been demonstrated in recent studies that a high CAC score is linked to the history and progression of congestive heart failure.30,31 While our study did not involve patients with overt heart failure, the association between CAC score and LVDD could indicate the occurrence of heart failure events in this population. As LVDD is one of the contributing factors to heart failure, incorporating the CAC score into the risk stratification for heart failure prediction may be beneficial. However, further studies are required to investigate this topic.
Study Limitations
Our analysis has several limitations. First, this study included patients with mixed symptomatic and asymptomatic presentations and the sample size was relatively small. Additionally, the study population predominantly consisted of women. Therefore, the generalisability of our results to the broader population or patients at higher risk may be limited. Second, all analyses were cross-sectional, which complicates the causal interpretation of the associations that were observed. Third, patients with a history of CAD, post percutaneous coronary intervention, coronary artery bypass surgery or AF were excluded from our analysis due to the potential for inaccurate CAC scores and significant artefact from cardiac motion.
Conclusion
This study highlights a significant association between CAC and LVDD in patients with hypertension without CAD. Patients with LVDD had higher CAC scores, and CAC independently predicted LVDD. 
Clinical Perspective
- Left ventricular diastolic dysfunction and coronary artery calcification are common in patients with hypertension.
- Coronary artery calcium score is independently associated with left ventricular diastolic dysfunction even after adjusting for traditional cardiovascular risk factors.