Randomised controlled trials have shown the safety and efficiency of transcatheter aortic valve implantation (TAVI) for the treatment of severe symptomatic aortic stenosis (AS) in patients across all surgical risks, even in those with low risk for open surgery.1,2 Consequently, the number of patients treated with TAVI has increased exponentially in recent times, including patients who are younger and with a low risk for surgery. Hence, with the increased life expectancy of younger patients after TAVI treatment, it is important to consider the lifetime management of patients beyond the initial TAVI procedure. One such important consideration is the preservation of coronary artery access after TAVI for potential future coronary angiograms (CA) and percutaneous coronary intervention (PCI).
Coronary artery disease (CAD) often coexists with severe AS in patients being considered for TAVI, with the incidence of CAD as high as 75%. Although optimal management of CAD before TAVI is controversial, PCI is recommended for severely stenosed proximal coronary lesions that subtend a large myocardial territory.3,4 CA and PCI after TAVI may be performed either due to progression of CAD or the development of acute coronary syndrome (ACS), particularly when in the subset of younger AS patients treated with TAVI who may have a higher lifetime risk for CAD progression.
The aims of this paper are to review the current published literature on CA/PCI success rates after TAVI, define factors leading to difficult CA/PCI after TAVI and provide a practical guide on optimising coronary access after TAVI.
Published Data on Coronary Access and Percutaneous Coronary Intervention After Transcatheter Aortic Valve Implantation
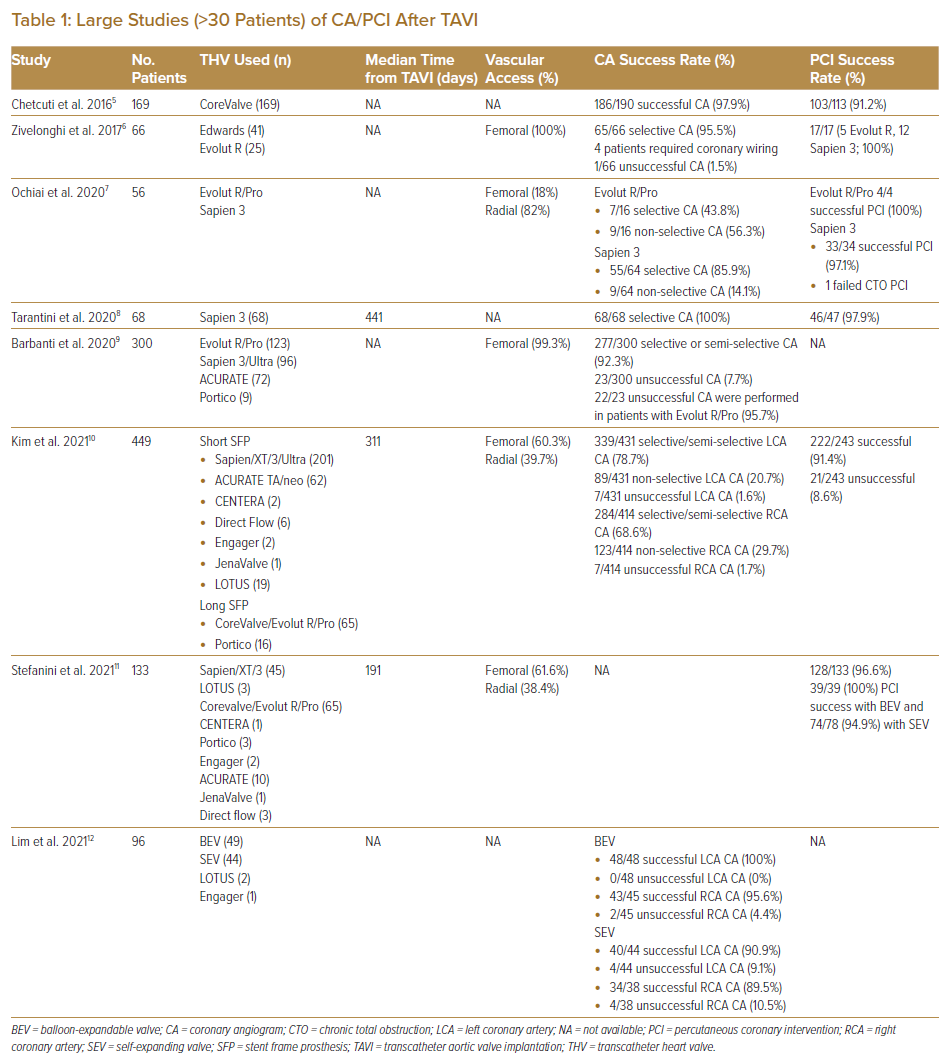
To date, only observational studies and registry data have been published on the feasibility of CA and PCI after TAVI (Table 1).5–12 The RE-ACCESS study by Barbanti et al. was a single-centre prospective registry-based study that enrolled 300 patients who underwent CA before and after TAVI using all commercially available devices (123 patients with Evolut R/Pro, 96 patients with Sapien 3/Ultra, 72 patients with ACURATE and nine patients with Portico).9 Successful coronary cannulation was high (92.3%). Unsuccessful coronary cannulation was found almost exclusively with the use of Evolut R/Pro devices (22 of 23 unsuccessful coronary cannulations). Independent predictors of unsuccessful coronary cannulation after TAVI were the use of Evolut R/Pro, a high TAVI to sinus of Valsalva (SoV) relationship and reduced implantation depth of TAVI devices.9
The REVIVIAL study reported 133 patients who underwent unplanned PCI after TAVI.11 The highest incidence of PCI was during the first week after TAVI and declined over time. The main indication within the first 2 years after TAVI was ACS, whereas chronic coronary syndromes were the main indication for PCI beyond 2 years. A high success rate (96.6%) for these unplanned PCIs was reported, with no significant differences between patients treated with balloon-expandable and self-expandable TAVI devices (100% versus 94.9%; p=0.150).11
Kim et al. reported from an international registry involving 25 centres on the feasibility of CA or PCI in the acute setting after TAVI, including a total of 449 patients.10 Even when performed in an urgent or emergency setting, success rates were high for CA (98.3% for right coronary artery [RCA] and 99.3% for left coronary artery [LCA]). TAVI devices were classified as either short stent-frame prosthesis (SFP), which included Sapien/XT/3/Ultra (n=201), ACURATE TA/neo (n=62), CENTERA (n=2), Direct Flow (n=6), Engager (n=2), JenaValve (n=1) and LOTUS (n=19), or long SFP, which included CoreValve/Evolut R/Pro (n=65) and Portico (n=16). The success rate for CA was higher in patients with short rather than long SFP for CA of RCA (99.6% versus 95.9%; p=0.005), but similar for both groups for CA of the LCA (99.7% versus 98.7%; p=0.24). The PCI success rate was 91.4% and did not differ between patients with short or long SFP (90.4% versus 93.4%; p=0.44).10
In a report from seven Asian and European centres that included 96 post-TAVI patients who underwent CA, a high (92%) success rate of CA was found.12 The use of self-expandable supra-annular valves (SESAV), increased valve size and increased oversizing for area were identified as risk factors for difficult CA.12 That report illustrated that CA can be performed in the vast majority of Asian patients with a success rate similar to that reported in non-Asian patients despite smaller aortic root and SoV dimensions.
These published data suggest that CA and PCI can be successfully performed in most patients. Nevertheless, certain TAVI valves pose greater challenges to coronary artery access after TAVI, particularly SESAV.
Factors Determining Ease of Coronary Access After Transcatheter Aortic Valve Implantation
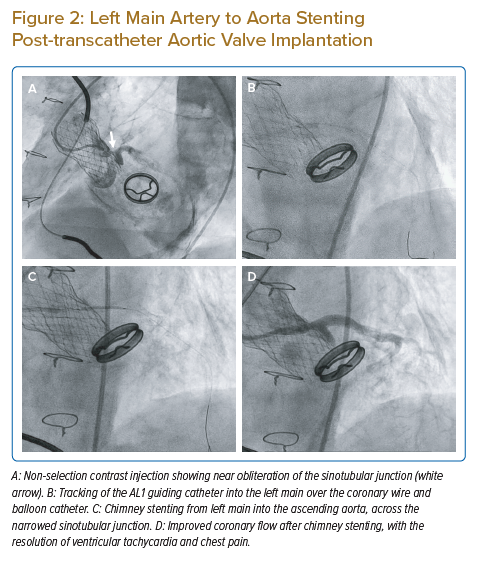
Factors that affect the success of CA/PCI after TAVI are similar to those that cause acute coronary artery occlusion during the index TAVI procedure. These factors are listed in Table 2 and can be summarised into anatomical, device and procedural factors.13 The presence of bulky aortic valve leaflets with a narrow SoV and low coronary artery height on multidetector CT (MDCT) increases the risk of coronary occlusion during the index TAVI procedure, which subsequently poses difficulties for coronary access after TAVI. Figure 1 shows an attempted angiogram in a patient with a prior LOTUS TAVI valve implantation. The LOTUS valve frame has extended beyond the covered sinotubular junction (STJ), making catheter passage from the top of the valve frame to the coronary sinus impossible. The open cells of the LOTUS valve were also too small to allow passage of the catheter. In this situation, selective CA and PCI were impossible.
When implanting TAVI within a failing aortic bioprosthetic valve replacement (valve-in-valve procedure), additional consideration should be given to the design of the failing bioprosthetic valve. Reports have been published of bioprosthetic valves with externally mounted leaflets (e.g. Mitroflow, Sorin and Trifecta [St Jude Medical] valve replacements) having an increased risk of coronary occlusion with such valve-in-valve procedures.14
MDCT performed as part of preprocedural planning can identify patients who are at higher risk of coronary occlusion during TAVI deployment or in whom coronary access after TAVI is likely to be difficult. Preprocedural planning software such as 3mensio (Pie Medical Imaging) enables the analysis of native aortic and coronary anatomy for the high-risk features stated above. It also allows for the projection of a virtual transcatheter heart valve (THV) to help predict the risk of coronary occlusion. The virtual heart valve to coronary (VTC) ostial distance can help predict the risk of coronary occlusion in valve-in-valve procedures, with a VTC distance of <4 mm predicting a high risk of coronary occlusion. The use of MDCT after TAVI will allow for a better understanding of the orientation of the TAVI valve commissures relative to the coronary ostium. Selective coronary cannulation will likely be difficult if the commissures are found to lie in front of the coronary ostium.
The upward displacement of the leaflets of the failed aortic bioprosthesis by the TAVI frame creates a ‘neoskirt’ consisting of displaced leaflets forming a sealed cylinder over the TAVI frame. This may result in sinus sequestration by the neoskirt, particularly if the length of the displaced leaflets exceeds that of the SoV height, and may make coronary reaccess impossible. The risk of sinus sequestration can be assessed by measuring the valve-to-STJ (VTSTJ) distance on MDCT. A narrow VTSTJ <2.5–3.5 mm is considered high risk for sinus sequestration.15 Tang et al. proposed the VIVID classification to stratify the risk of coronary occlusion and sinus sequestration with aortic valve-in-valve procedures.16 This classification takes into consideration extension of the failed bioprosthesis leaflet above or below the coronary ostium plane, the width of the SoV, and the width and height of the STJ. Patients determined to be at high risk of coronary occlusion or sinus sequestration where the aortic valve leaflet extends above the STJ with effaced sinuses (type IIIb) or with narrow STJ (type IIIc) may be considered for procedures such as the bioprosthetic or native aortic scallop intentional laceration to prevent iatrogenic coronary artery obstruction (BASILICA) procedure.16 This is discussed in greater detail below.
The design of the TAVI prosthesis to be implanted affects the ease of coronary artery access, and this should be taken into consideration when choosing the TAVI prosthesis, particularly in younger patients, to maintain coronary access. In general, a shorter TAVI prosthesis frame using the balloon-expandable valve (BEV; e.g. Sapien THV; Edwards Lifesciences) usually poses less difficulty for coronary reaccess. This is in contrast with the SESAV (e.g. Medtronic CoreValve), which has a longer prosthesis valve frame, supra-annular valve leaflet position and a smaller frame cell size, posing greater difficulties for coronary reaccess. A summary of the salient features of commonly used current-generation TAVI prosthesis that affect coronary access after TAVI and best practice recommendations are presented in Table 3.13,17,18
Optimising Coronary Access During the Index Transcatheter Aortic Valve Implantation
Preprocedural planning can identify patients with a higher likelihood of requiring coronary access after TAVI, including younger patients, patients with multiple poorly controlled CAD risk factors, patients with high SYNTAX scores and those with a prior PCI. MDCT performed as part of TAVI procedural planning can identify anatomical factors, as above, that increase the difficulty of coronary artery reaccess. When such patients are identified, consideration should be given to selecting a THV with a short valve frame, intra-annular leaflet position and large THV cells to allow for easier coronary access after TAVI. In such patients, extra care must be taken during the procedure to avoid an excessively high THV implant position and to orientate commissure tabs away from the coronary ostium.
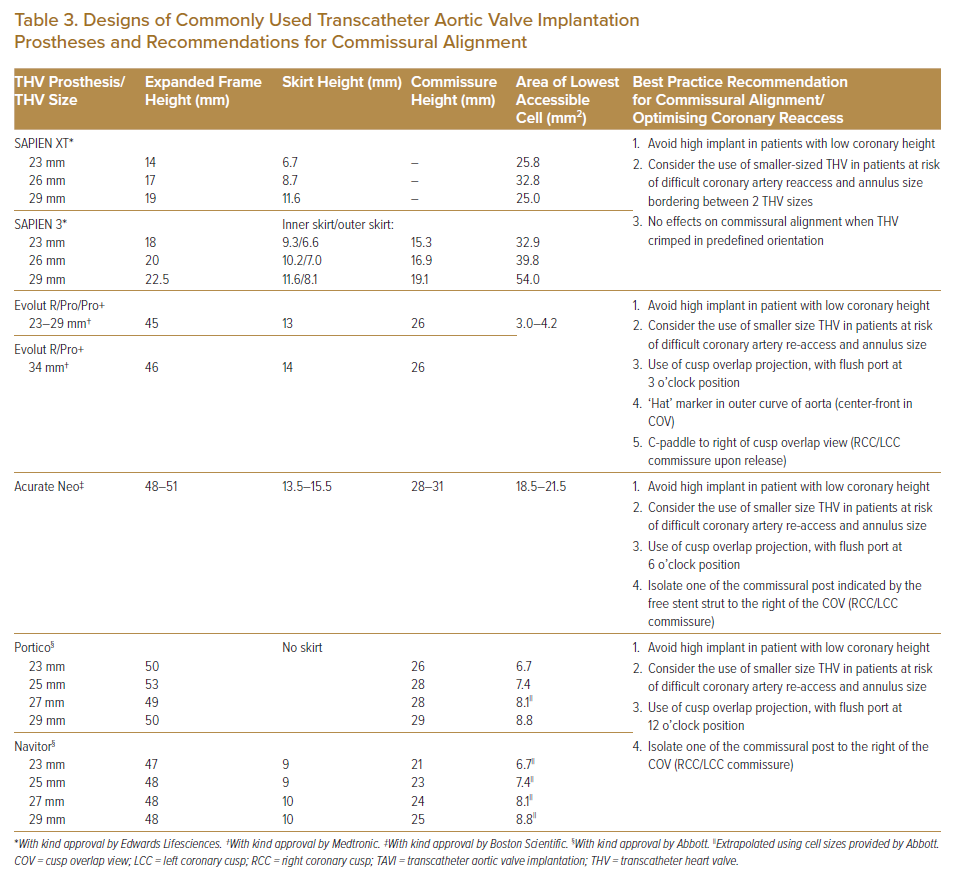
Procedural steps taken to align TAVI valve commissures away from the coronary ostial have been described with improved rates of successful coronary reaccess after TAVI compared to no commissural alignment.
Tang et al. found no effects on coronary commissure alignment when BEVs were crimped in predefined orientation before the deployment.19 The same group described how commissural alignment could be achieved with the SESAV to improve coronary reaccess. The delivery of the SESAV is performed with the flush port of the delivery catheter facing the 3 o’clock position. This manoeuvre aims to position the ‘Hat’ marker of the SESAV on the outer course of the ascending aorta. Deployment in this manner will isolate the commissure tab between the right coronary cusp (RCC) and left coronary cusp (LCC). This can be confirmed by the ‘Hat’ marker of the SESAV in the centre front position in the cusp overlap view, where the RCC and LCC overlap on fluoroscopy. The C-tab will also be seen on the right of the screen in this cusp overlap view. With commissural alignment, the incidence of coronary overlap was significantly reduced for the LCA from 31.4% to 18.7% (p=0.33) and for the RCA from 20.7% to 11.2% (p=0.047) compared with no commissural alignment.19
Similarly, in the prospective COMALIGN study, Bieliauskas et al. described the commissural alignment of the CoreValve Evolut, ACURATE Neo 2 and Portico self-expanding THVs isolating the commissural tab between the RCC and LCC in the cusp overlap view by keeping the flush port positioned at the 3 o’clock (Evolut), 6 o’clock (ACURATE) and 12 o’clock (Portico) positions when delivering the valve system to the aortic annulus.20 With these manoeuvres, mild commissural misalignment (<30º) was obtained in 88% of the cohort, with 60% of patients having optimal (<15º) commissural alignment.20
The ALIGN-ACCESS study evaluated the impact of commissural alignment on the feasibility of CA after TAVI.21 In that study, 206 patients were evaluated, with a Sapien 3 valve used in 38%, the Evolut Pro/R used in 31.1% and the ACURATE Neo used in 30.1%. Commissural alignment was attempted and found favourable for 85.9% of patients with the Evolut and 69.4% of patients with the ACURATE Neo. Selective CA was higher for SAPIEN 3 than for aligned and misaligned SESAV (95% versus 71% versus 46%, respectively; p<0.001).21 This demonstrated that, with commissural alignment, the rate of selective CA after TAVI improved for SESAV. A lower volume of contrast medium and shorter fluoroscopic time were found in CA procedures performed for aligned compared with misaligned SESAV.21 However, there is greater risk of unfeasible CA even with aligned SESAV compared with the short-frame SAPIEN 3. Misaligned SESAV, short SoV height and a low ratio of THV diameter to SoV diameter were found to be independent predictors of unsuccessful CA.21
The alignment of the leaflet commissures optimises coronary access after TAVI and increases the likelihood of coronary patency in future valve-in-valve procedures, particularly in younger patients. Nevertheless, in some patients who are at risk of coronary artery occlusion and difficult coronary reaccess, consideration should be given to the use of chimney stenting, where a coronary stent is positioned prophylactically in the coronary artery before TAVI deployment. The stent is pulled back and deployed from the coronary ostium into the ascending aorta after TAVI deployment to maintain patency of the coronary artery when there is compromised coronary artery flow. The primary drawback of this technique is difficulty with future reaccess.
An alternative to chimney stenting is the BASILICA technique. The BASILICA technique lacerates the aortic leaflets using an electrified coronary wire, allowing the lacerated leaflets to splay to the side, thus reducing the risk of coronary artery occlusion, and maintaining coronary access for repeat angiogram or valve-in-valve procedures in the future. A detailed description of the chimney stenting and BASILICA techniques is beyond the scope of this review.
Procedural Considerations for Coronary Angiograms and Percutaneous Coronary Intervention After Transcatheter Aortic Valve Implantation
In general, BEVs do not pose great difficulty in coronary reaccess due to their short valve frame, intra-annular valve leaflet position and large frame cell size to allow coronary artery catheters to pass. Standard diagnostic catheters can be used for CA and PCI without modification.
Procedural consideration will henceforth be focused on longer-frame self-expandable valves (SEV), particularly SESAV. The longer valve frames, supra-annular leaflet position and smaller frame cell sizes (compared with the BEV) of the SEV pose additional challenges when attempting to selectively cannulate the coronary ostium.
When performing CA in patients with prior SESAV implantation, right radial artery access remains the default vascular access for most patients. However, if difficulty is encountered with the right radial artery approach, either femoral artery or left radial artery access may reduce catheter manipulation and alter catheter angulation to achieve selective coronary cannulation. It may be challenging to deliver the J-tipped guidewire and catheters within the valve frame of the SEV, particularly in the presence of a horizontal aorta. In such situations, the guidewire may preferentially enter the space between the aorta and valve frame. Careful manipulation of the catheter and guidewire, often in orthogonal views, helps direct the catheter within the valve frame. The ability of the J-tipped guidewire and catheter to easily cross into the aortic valve further helps confirm the position within the valve frame. Once positioned within the valve frame, this position should be maintained using an exchange length guidewire during catheter exchange to reduce procedural time.
Unlike the shorter BEV, where engagement of the coronary ostium may be attempted over the superior edge of the valve frame, the longer SEV will require the engagement of the coronary ostium through the open cells of the valve frame. This poses a few challenges, including selecting the correct open cell closest to the coronary ostium and delivering the correctly shaped catheter for selective ostial engagement and angiography. The valve frame and constrained segment of the SESAV will reduce the size of the root of the ascending aorta compared with the native aortic root size. With this in mind, catheters to engage the left main (LM) ostium, with catheter support attained from the ascending aorta, should have a smaller secondary curve than those used to engage the left coronary ostia without TAVI prosthesis (e.g. using a left Judkins [JL] 3.5 catheter instead of a JL4 catheter to engage the LM). Second-line catheters for engagement of the left coronary ostium include right Judkins (JR) 4, Ikari right 1.5/1.0, or femoral left 3.0 catheters.13 Extra backup (EBU) guiding catheters should be used with caution for the left coronary ostium with a smaller EBU 3.0 guiding catheter used rather than a standard EBU 3.5 guiding catheter. A case report of entrapment of the EBU guiding catheters within the TAVI valve frame leading to coronary dissection and eventual periprocedural mortality has been published.22 For LCA catheters, standard JR4 catheters can be used as the initial catheter for engagement. Alternative catheters for engaging the right coronary ostium include the Ikari right 1.5, Multipurpose, and Amplatz right (AR) catheters.13 In difficult cases, a baseline aortogram may help with a better understanding of the position of the TAVI valve in relation to the coronary ostium. The catheters that are successfully used to engage coronary ostia after TAVI are often non-standard catheters (e.g. JR catheter for LM engagement and AR2 catheter for RCA engagement). Operators should have a low threshold to switch catheters if standard catheters fit poorly.
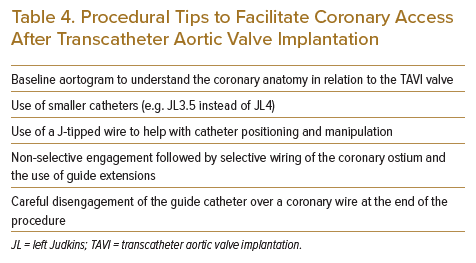
Once close to the target coronary ostium, non-selective sinus contrast injection or an ascending aortic root angiogram can help identify the target valve frame cell closest to the coronary ostium. The catheters can be straightened by Teflon J-tipped or stiff 0.035″ guidewires within the catheter and manipulated to engage through the open valve frame cell closest to the target coronary ostium. In more challenging cases, the hydrophilic Terumo angled tip guidewire can be used in place of the Teflon J-tipped wire to help select the correct open cell in the valve frame closest to the coronary ostium. Despite the use of the correct catheter size and shape, selective engagement of the coronary ostium may not be achievable, especially if the TAVI commissure is deployed in front of the coronary ostium, if bulky native leaflets are obstructing selective catheter engagement or if there is a combination of low coronary height and high TAVI prosthesis deployment. In such situations, wiring of the coronary artery can be attempted with the catheter brought as close to the ostium as possible.
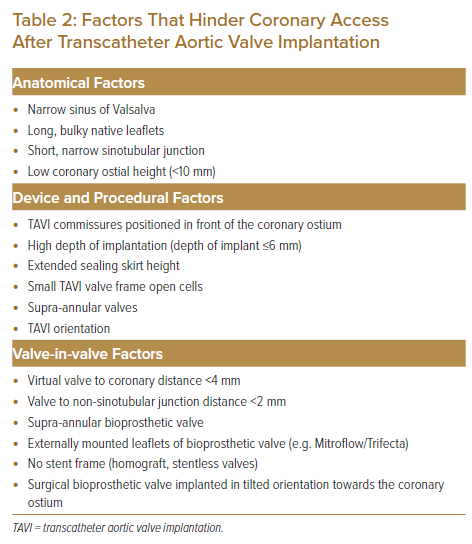
Once the coronary artery is successfully wired, this may provide enough support for the guide catheter to slide over the wire for selective coronary engagement. If this is not possible, two other manoeuvres may be considered. An attempt can be made to pass a rapid exchange balloon catheter to the coronary artery, and this can provide additional support for the guiding catheter to slide over and engage more selectively. If a coronary lesion is identified for PCI, the balloon catheter can be inflated over the lesion. While the balloon is inflated, the guiding catheter will receive additional support for selective engagement. Figure 2A shows a patient with chest pain and ventricular tachycardia 5 months after implantation of an SESAV. The SESAV implantation was deliberately positioned high in the index TAVI procedure to ensure that the SESAV did not affect the movement of a Björk–Shiley mitral valve replacement implanted a decade before the TAVI procedure. The high implant of the SESAV resulted in the sealing skirt of the SESAV almost obliterating the STJ. Multiple catheters were used without achieving selective coronary ostium cannulation. An AL1 guiding catheter with careful manipulation achieved the best position, next to the small opening in the STJ. From this position, a coronary wire was passed to the LM artery and left anterior descending (LAD) artery. The AL1 guiding catheter was able to track into the LM shaft with the support of the coronary wire and balloon catheter (Figure 2B). Chimney stenting was performed from the LM artery into the ascending aorta through the valve frame and the near-obliterated STJ with a drug-eluting stent (Figures 2C and 2D). Coronary flow in the LM/LAD improved after chimney stenting, with the resolution of ventricular tachycardia, chest pain and ECG changes.
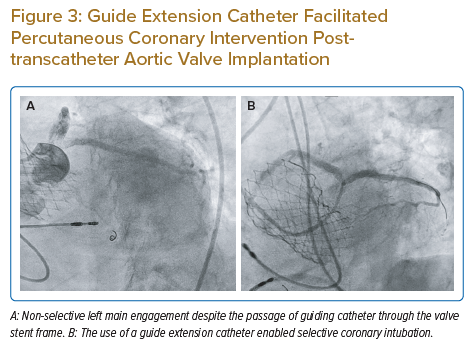
Alternatively, if the balloon catheter does not provide enough support for catheter engagement and there is no suitable side branch or lesion for the balloon to be inflated, a guide extension catheter can be used to selectively engage the coronary ostium via the guidewire and balloon catheter. The guide extension catheter is particularly useful both to help with device/stent delivery and for contrast opacification of the coronary arteries when PCI is required. Figure 3A illustrates the inability of the guiding catheter to engage the LM ostium selectively despite the passage of the catheter through the stent frame of the SESAV. At this position, the LM artery was successfully wired and a guide extension catheter was delivered into the LM shaft to enable selective LM engagement, adequate coronary opacification with contrast and completion of the PCI (Figure 3B).
Upon completion of the CA or PCI procedure, care should be taken to carefully disengage the coronary catheter from the coronary ostium. Entrapment of the catheter in the valve frame can occur, and excessive force should be avoided when removing the catheter through the frame. The disengagement and safe withdrawal of the catheter can usually be achieved by straightening the catheter using a J-tipped wire under fluoroscopy. Table 4 summarises some of these techniques.
Conclusion
With a higher proportion of younger patients treated with TAVI for symptomatic AS, the lifetime risk of requiring coronary reaccess for both diagnostic CA and PCI is increased in these patients. The maintenance and optimisation of coronary reaccess start with meticulous preprocedural planning, thoughtful TAVI prosthesis selection and careful TAVI prosthesis implantation to ensure commissural alignment, especially in the case of SESAV. When faced with the challenge of coronary reaccess in a patient with a TAVI prosthesis in situ, a good understanding of the patient’s aortic anatomy, TAVI prosthesis structure and corresponding modification of catheter selection and engagement will ensure that coronary reaccess is performed in a safe and optimised manner.
Clinical Perspective
- Coronary reaccess for angiography or intervention is possible for most patients after TAVI. Difficulties are encountered most with supra-annular self-expanding valves.
- Understanding various factors that hinder coronary reaccess and modifications of standard interventional techniques can help with adequate coronary artery opacification with contrast and successful coronary intervention when required.